Abstract
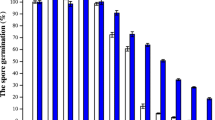
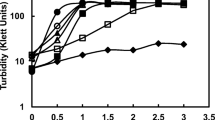
Dipicolinic acid synthesis inPenicillium citreoviride strain 3114 was inhibited by Ca2+ ions, but not by Ba2+, Cu2+or Fe2+. Among the metals tested, only Zn2+ inhibited the synthesis of dipicolinic acid and promoted sporulation. None of these metals reversed the inhibition by Ca2+ or Zn2+. A mutant 27133-dpa-ca selected for resistance to feedback inhibition by dipicolinic acid: Ca2+ complex showed cross-resistance to inhibition by dipicolinic acid: Zn2+. Both 3114 and271 33-dpa-ca excreted a number of aliphatic and amino acids during secondary metabolism of dipicolinic acid. In the presence of 1000 ppm of Ca2+, accumulation of citric acid and α-aminoadipic acid was completely inhibited under conditions of inhibition of dipicolinic acid in parent strain 3114 but not in the mutant. Citric acid with or without Ca2+ did not inhibit thede novo synthesis of dipicolinic acid in the strain 3114. In fact, citric acid in the presence of Ca2+ improved significantly rate of dipicolinic acid synthesis. Apart from resistance to feed back inhibition by dipicolinic acid: Ca2+ complex, mutant differed from the parent in three other aspectsviz. (i) dipicolinic acid synthesis was not subject to catabolite repression by glucose, (ii) sporulation as well as dipicolinic acid synthesis was dependent on the presence of Ca2+ ions in the medium and (iii) Mg2+ requirement for the mutant increased three fold. Higher requirement of the Mg2+ could be partially relieved by Ca2+ during secondary metabolism. The results support the inference thatde novo synthesis of dipicolinic acid is regulated through feedback inhibition by dipicolinic acid: Ca2+complex.
Similar content being viewed by others
Abbreviations
- DPA:
-
Dipicolinic acid -2,6-dicarboxylic acid
- NTG:
-
nitrosoguanidine
References
Cameron, L. E. and Le John, J. (1972)J. Biol Chem.,247,4729.
Demain, A. L. (1966)Adv. Appl. Microbiol.,8, 1.
Forman, M. and Aronson, A. (1972)Biochem. J.,126, 503.
Gould, G. W. and Dring, G. J. (1974)Adv. Microb. Physiol.,11,137.
Hackette, S. L, Skyl, G. E., Burton, C. and Segel, I. K. (1970)J. Biol. Chem.,245,4241.
Kalle, G. P. (1978) inPerspectives in Industrial Microbiology, eds. G. P. Kalle, Y. M. Freitas and D. V. Tamanhe (Asssociation of Microbiologists of India) p. 125.
Kalle, G. P and Khandekar, P. S. (1983)J. Biosci.,5,43.
McArdle, B. (1955)Biochem. J.,60,647.
Murrel, W. G. (1967)adv. Microb. Physiol.,1,33.
King, K., Gross, W. and Heinz, E (1970)Arch. Biochem. Biophys.,137,243.
Schwanz, D. P. (1963) inHandbook of Analytical Chemistry, ed. L. Meites (NewYork: McGraw Hill) p. 644.
Tamir, H. and Gilvarg, C. (1974)J. Biol. Chem.,249,3034.
Tanenbaum, S. W. and Kaneko, K. (1964)Biochemistry.,3,1314.
Weinberg, E. D. (1970)Adv. Microb. Physiol.,4,1.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kalle, G.P., Deo, Y.M. Effect of calcium on synthesis of dipicolinic acid inPenicillium citreoviride and its feedback resistant mutant. J Biosci 5, 321–330 (1983). https://doi.org/10.1007/BF02716698
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02716698