Abstract
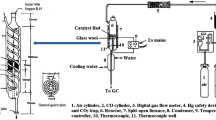
Mo-Cr-V-Bi-Si multi-component oxide catalysts were synthesized by three different coprecipitation methods and used in the controlled oxidation of methane to methanol and formaldehyde. It was shown that Mo content in Mo-V-Cr-Bi-Si oxides and the performance of these catalysts were strongly influenced by different coprecipitation methods. The highest methanol and formaldehyde selectivity of 80.2% could be achieved at a methane conversion of 10 % for the catalyst prepared by a particular method. The results of XRD indicated that the crystalline phase structures of catalysts were sensitive to Mo, V and Bi loadings. Bi(III) could combine with V(V) and Mo(VI) to form BiVO4 and γ-Bi2MoO6, whereas Cr seemed to form a single Cr2O2 crystalline phase in the presence of Bi. The effects of Mo and Cr loading on controlled methane oxidation were also investigated. Mo(VI) oxide appears to favor the formation of partial oxidation products and Cr(III) oxide seems to enhance the conversion of methane.
Similar content being viewed by others
References
Barbaux, Y., Elamrani, A. R., Payen, E., Gengembre, L., Bonnelle, J. P. and Grzybowska, B., “Silica Supported Molybdena Catalysts Characterization and Methane Oxidation”,Appl. Catal.,44, 117 (1988).
Brown, M. J. and Parkyns, N. D., “Progress in the Partial Oxidation of Methane to Methanol and Formaldehyde”,Catal. Today,8, 305 (1991).
Chen, S. Y. and Willcox, D., “Effect of Vanadium Oxide Loading on the Selective Oxidation of Methane over V2O5/ SiO2”,Ind. Eng. Chem. Res.,32, 584 (1993).
Faraldos, M., Banares, M. A., Anderson, J. A., Hu, H., Wachs, I. E. and Fierro, J. L., “Comparison of Silica-Supported MoO3 and V2O5 Catalysts in the Selective Partial Oxidation of Methane”,J. Catal.,160, 214 (1996).
Foster, J. H., “Direct Catalytic Oxidation of Methane to Methanol”,Appl. Catal.,19, 11 (1985).
Fox, J. M., Chen, T. P. and Degen, B. D., “An Evaluation of Direct Methane Conversion Processes”,Chemical Engineering Progress, April, 42 (1990).
Han, Z. S., Pan, W., Li, J. L. and Zhu, Q. M., “The Effect of Vanadium and Bismuth Promoter on Direct Partial Oxidation of Methane”,Tsinghua Sci. and Tech.,1, 42 (1996).
Iwamoto, M., Yoda, Y., Yamazoe, N. and Selyama, T., “Study of Metal Oxide Catalysts by Temperature Programmed Desorption. 4. Oxygen Adsorption on Various Metal Oxides”,J. Phys. Chem.,82(24), 2564 (1978).
Iwamoto, M., Japanese Patent 58 92,629 1983a.
Iwamoto, M., Japanese Patent 58 92,639 1983b.
Pitchai, R. and Klier, K., “Partial Oxidation of Methane”,Catal. Rev. -Sci. Eng.,28(1), 13 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Han, Zs., Pan, W., Pan, Wx. et al. Preparation and effect of Mo-V-Cr-Bi-Si oxide catalysts on controlled oxidation of methane to methanol and formaldehyde. Korean J. Chem. Eng. 15, 496–499 (1998). https://doi.org/10.1007/BF02707098
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02707098