Abstract
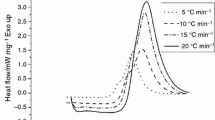
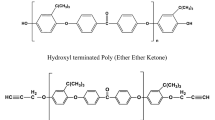
The cure kinetics of blends of epoxy resin (4,4’-tetraglycidyl diaminodiphenyl methane; TGDDM)/curing agent (diaminodiphenyl sulfone; DDS) with ATPEI (amine terminated poly-etherimide) -CTBN (carboxyl terminated poly (butadiene-co-acrylonitrile)) block copolymer (AB type) were studied using differential scanning calorimetry under isothermal conditions to determine the reaction kinetic parameters such as activation energy and reaction constants. Final cure conversion decreased with increasing amount of AB in the blends. A diffusion controlled reaction was observed as the cure conversion increased, and the curing reaction was successfully analyzed by incorporating the diffusion control term in the rate equation for the epoxy/DDS/AB blends. The fracture toughness was improved to about 350% compared to that of the unmodified resin at 30% of AB block copolymer. This is attributed to the formation of co-continuous morphology between the epoxy phase and AB block copolymer phase. By increasing the amount of AB, the modulus of the cured blends decreased, which was due to the presence of CTBN rubbery phases.
Similar content being viewed by others
References
Agag, T. and Takeichi, T.,“Synthesis and Characterization of Epoxy Film Cured with Reactive Polyimide,”Polymer,40, 6557 (1999).
Aling, I. and Jenniger, W. F.,“Curing Kinetics of Phase Separating Epoxy Thermosets Studied by Dielectric and Calorimetric Investigations; A Simple Model for the Complex Dielectric Permittivity,”Polymer,36, 246 (1998).
Bonnet, A., Pascault, J. P., Sautereau, H., Taha, M. and Camberlin, Y.,“Epoxy-diamine Thermoset/Thermoplastic Blends. 1. Rates of Reactions Before and After Phase Separetion,”Macromolecules,32, 8517 (1999).
Bonnet, A., Pascault, J. P., Sautereau, H. and Camberlin, Y.,“Epoxy-Diamine Thermoset/Thermoplastic Blends. 2 Rheological Behavior Before and After Phase Separation,”Macromolecules,32, 8524 (1999).
Bucknall, C. B. and Gilbert, A. H., “Toughening Tetrafunctional Epoxy Resins using Polyetherimide,”Polymer,30, 213 (1989).
Chen, D., Pascault, J. P., Bersch, R. S. and Sieboert, A. R.,“Synthesis, Characterization and Properties of Reactive Liqid Rubbers Based on Butadiene-acrylonitrile Copolymers,”J. Appl. Polym. Sci.,51, 1959 (1994).
Chern, C. S. and Poehlein, G W.,“Kinetic Model for Curing Reactions of Epoxides with Amines,”Polym. Engng. Sci.,27, 782 (1987).
Girard-Reydet, E., Sautereau, H. and Pascault, J. P.,“Polyetherimide-Modified Epoxy Networks: Influence of Cure Conditions on Morphology and Mechanical Properties,”Polymer,40, 1677 (1999).
Hourston, D. J. and Lane, J. M.,“The Toughening of Epoxy Resins with Thermoplastics. 1. Trifunctional Epoxy Resin-polyetherimide Blends,”Polymer,33, 1379(1992).
Keenan, M. R.,“Auto Catalytic Cure Kinetics from DSC Measurements: Zero Initial Cure Rate,”J. Appl. Polym. Sci.,33, 1725 (1987).
Kim, M., Kim, W., Choe, Y., Ahn, B., Kim, D.-S. and Park, J.-M.,“Modeling of Multi-Autocatalytic Cure Reactions of an Epoxy/Amine Terminated Polyetherimide/NMA System,”Polymer Bulletin,51, 167 (2003).
Lopez, J., Lopez-Bueno, I., Nogueira, P., Ramirez, C., Abad, M. J., Barral, L. and Cano, J.,“Effect of Poly(styrene-co-acrylonitrile) on the Curing of an Epoxy/amine Resin,”Polymer 42, 1669 (2001).
Mimura, K., Ito, H. and Fujioka, H.,“Improvement of Thermal and Mechanical Properties by Control of Morphologies in PES-Modified Epoxy Resins,”Polymer,41, 4451 (2000).
Oyanguren, P. A., Riccardi, C. C., Williams, R. J. J. and Mondragom, I.,“Phase Separation Induced by a Chain Polymerization: Polysul-fone-Modified Epoxy/Anhydride Systems,”Polymer,36, 1349 (1998).
Rahagopalan, G., Immaordino, K. M., Gillespie, J. W. and McKnight, S. H.,“Diffusion and Reaction of Epoxy and Amine in Polysulfone Studied using Fourier Transform Infrared Spectroscopy: Experimental Results,”Polymer,41, 2591 (2000).
Ryan, M. E. and Dutta, A.,“Kinetics of Epoxy Cure: A Rapid Technique for Kinetic Parameter Estimation,”Polymer,20, 203 (1979).
Sourour, S. and Kamal, M.R.,“Differential Scanning Calorimetry of Epoxy Cure: Isothermal Cure Kinetics,”Thermochin Acta,14, 41 (1976).
Su, C. C. and Woo, E. M.,“Cure Kinetics and Morphology of Aminecured Tetraglycidyl-4,4-diaminodiphenylmethane Epoxy Blends with Poly(ether imide),”Polymer,36, 2883 (1995).
Varley, R. J., Hodgkin, J. H., Hawthorne, D. G., Simon, G P. and Maculloch, D.,“Toughening of a Trifunctional Epoxy System Part III; Kinetic and Morphological Study of the Thermoplastic Modified Cure Process,”Polymer,41, 3425 (2000).
Wise, C. W., Cook, W D. and Goodwin, A. A.,“CTBN Rubber Phase Precipitation in Model Epoxy Resins,”Polymer,41, 4625 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D., Beak, Jo., Choe, Y. et al. Cure kinetics and mechanical properties of the blend system of epoxy/diaminodiphenyl sulfone and amine terminated polyetherimide-carboxyl terminated poly(butadiene-co-acrylonitrile) block copolymer. Korean J. Chem. Eng. 22, 755–761 (2005). https://doi.org/10.1007/BF02705795
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705795