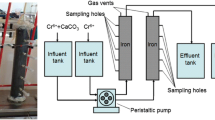
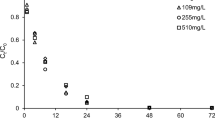
Abstract
Use of reduced metals has attracted much attention since it possesses a great potential for eliminating reducible contaminants in groundwater such as heavy metals and chlorinated compounds. However, products of metal-mediated reactions for many chlorinated hydrocarbons have not clearly been identified. In addition, consumption of the metals, generation and release of metal ions, formation of insoluble metal oxides and hydroxides on the clean metal surface, and rise of pH inevitably accompany the reactions. Due to these properties of metal-mediated reactions, the reaction rate could decrease as the reaction proceeds, and effluent quality could decay. It was shown in this study using chlorine mass balance and GC analysis that chloroform is formed from carbon tetrachloride by reduced iron. It is also well-known that nitrate is reduced mostly to ammonia by metals, which indicates that the metal process is inappropriate for denitrification of nitrate-contaminated aquifers. These results indicate that groundwater remediation using metal process requires careful consideration for the safety of reaction products. It was also shown that mixing rate strongly affects reaction rate since metal-mediated reaction occurs on the surface of metals. In addition, reaction rate was decreased due to metal hydroxide deposition on the surface of metal granules that was seen by scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis. Generation of iron ions (consumption of reduced iron) released from reduction of zero-valent iron was also shown by using an ion chromatograph (IC). In this study, some methods were suggested to solve the above-mentioned problems. Acid washing appeared effective for removing corrosion products on the surface of metal granules, by which a reduction rate could be maintained high for an extended time of reaction. Use of iron sulfide decreased an extent of pH rise during metal-mediated reaction; thereby precipitation of insoluble metal (hydr)oxides is expectedly decreased. It was also shown that inexpensive iron scrap instead of fine metal powders can be used for metal processes.
Similar content being viewed by others
References
Agrawal, A. and Tratnyek, P. G., “Reduction of Nitroaromatic Compounds by Zero-Valent Iron Metal,”Environ. Sci Technol,30, 153 (1996).
Benner, S. G., Blowes, D. W., Gould, W. D., Herbert, R. B. and Ptacek, C. J., “Geochemistry of a Permeable Reactive Barrier for Metals and Acid Mine Drainage,”Environ. Sci. Technol.,33, 2783 (1999).
Blowes, D. W., Ptacek, C. J. and Jambor, J. L., “In-Situ Remediation of Chromate Contaminated Groundwater Using Permeable Reactive Walls,”Environ. Sci. Technol.,31, 3348 (1997).
Blowes, D. W., Ptacek, C. J., Benner, S. G, McRae, C. T., Bennett, T. A. and Puls, R. W., “Treatment of Contaminants Using Permeable Reactive Barriers,”J. Contaminant Hydrol.,45, 123 (2000).
Burris, D. R., Campbell, T. I. and Manoranjan, V. S., “Sorption of Trichloroethylene and Tetrachloroethylene in a Batch Reactive Metallic Iron-Water System,”Environ. Sci. Technol.,29, 2850 (1995).
Butler, E. C. and Hayes, K.F., “Effect of Solution Composition and pH on the Reductive Dechlorination of Hexachloroethane by Iron Sulfide,”Environ. Sci. Technol.,32, 1276 (1998).
Chang, W D., Karra, S. B. and Senkan, S. M., “Molecular Beam Mass Spectroscopic Study of Trichloroethylene,”Environ. Sci. Technol.,20, 1243 (1986).
Chen, J. L., Al-Abed, S. R., Ryan, J. A. and Li, Z., “Effect of pH on Dechlorination of Trichloroethylene by Zero-Valent Iron,”J. Hazard. Mater.,B83, 243 (2001).
Choe, S., Chang, Y. Y, Hwang, K. Y. and Khim, J., “Kinetic of Reductive Denitrification by Nanoscale Zero-Valent Iron,”Chemosphere,41, 1307 (2000).
Deng, B., Burris, D. R. and Campbell, T. J., “Reduction of Vinyl Chloride in Metallic Iron-Water Systems,”Environ. Sci. Technol.,33, 2651 (1999).
Dolan, M. E. and McCarty, P. L., “Small-Column Microcosm for Assessing Methane-Stimulated Vinyl Chloride Transformation in Aquifer Samples,”Environ. Sci. Technol.,29, 1892 (1995).
Fogler, S., “Element of Chemical Reaction Engineering,” 2nd ed., Prentice Hall, Englewood Cliffs (1992).
Fox, B. G., Froland, W A, Jollie, D. R. and Lipscomb, J. D., “Haloalkene Oxidation by the Soluble Methane Monooxygenase fromMethylosinus trichosporium OB3b: Mechanistic and Environmental Implications,”Biochemistry,29, 6419 (1990).
Gillham, R. W. and O’Hannesin, S. T., “Enhanced Degradation of Halogenated Aliphatics by Zero-Valent Iron,”Groundwater,32, 958 (1994).
Gillham, R. W. and O’Hannesin, S. T, “Field Applications of Metal Enhanced Dehalogenation of Chlorinated Organic Contaminants,” Proceedings of WEFTEC 95, 68th Annual Conference and Exposition,2, 507 (1995).
Glod, G, Angst, W, Holliger, C. and Schwarzenbach, R. P., “Corrinoid-Mediated Reduction of Tetrachloroethene, Trichloroethene, and Trichlorofluoroethene in Homogeneous Aqueous Solution: Reaction Kinetics and Reaction Mechanisms,”Environ. Sci. Technol.,31, 253 (1997).
Gotpagar, J., Lyuksyutov, S., Cohn, R., Grulke, E. and Bhattacharyya, D., “Reductive Dehalogenation of Trichloroethylene with Zero-Valent Iron: Surface Profiling Microscopy and Rate Enhancement Studies,”Langmuir,15, 8412 (1999).
Gu, B., Phelps, T. J., Liang, L., Dickey, M. J., Yin, X. and Dai, S., “Reductive Precipitation of Uranium (VI) by Zero-Valent Iron,”Environ. Sci. Technol.,32, 3366 (1998).
Gu, B., Phelps, T J., Liang, L., Dickey, M. J., Roh, Y, Kinsall, B., Palumbo, A. V. and Jacobs, G K., “Biogeochemical Dynamics in Zero-Valent Iiron Columns: Implications for Permeable Reactive Barriers,”Environ. Sci. Technol.,33, 2170 (1999).
Haarstrick, A., Kut, O. M. and Heinzle, E., “TiO2-Assisted Degradation of Environmentally Relevant Organic Compounds in Wastewater Using a Novel Fluidized Bed Photoreator,”Environ. Sci Technol.,30, 817 (1996).
Hua, I. and Hoffmann, M. R., “Kinetics and Mechanism of the Sonolytic Degradation of CCI4: Intermediates and Byproducts,”Environ. Sci Technol.,30, 864 (1996).
Hung, H. M. and Hoffmann, M. R., “Kinetics and Mechanism of the Enhanced Reductive Degradation of CC14 by Elemental Iron in the Presence of Ultrasound,”Environ. Sci. Technol.,32, 3011 (1998).
Kielemoes, J., Boever, P. D. and Verstraete, W., “Influence of Denitrification on the Corrosion of Iron and Stainless Steel Powder,”Environ. Sci. Technol.,34, 663 (2000).
Kong, S. H., Kwon, C. I. and Kim, M. H., “Ozone Kinetics and Diesel Decomposition by Ozonation in Groundwater,”Korean J. Chem. Eng.,20, 293 (2003).
Mackay, D. M. and Cherry, J. A., “Groundwater Contamination: Pump- and-Treat Remeadiation,”Environ. Sci. Technol.,23, 630 (1989).
Mackay, D. and Shiu, W. W., “Henry’s Law Constants of Selected Aromatic Hydrocarbons, Alcohols, and Ketones,”J. Chem. Eng. Data,42, 27 (1997).
Matheson, L. J. and Tratnyek, P. G., “Reduction of Nitrate and Nitrite by Iron Metal; Implications for Groundwater Remediation,” Extended Abstract, American Chemical Society, San Francisco, CA, 13–17 April (1994).
Mok, Y S., Kang, H C. and Cho, M. H., “Oxidation of Volatile Organic Compounds by Using a Microwave-Induced Plasma Process,”Korean J. Chem. Eng.,20, 239 (2003).
MSE Technology Applications, “Subsurface Barriers Monitoring and Verification Technologies“ Report for U.S. Department of Energy (TTP #PE1-5-10-06) (1995).
NAS, “Alternatives for Ground Water Cleanup. Report of the National Academy of Science Committee on Ground Water Cleanup Alternatives,” National Academy Press, Washington, DC. (1994).
Palmer, P. L., “Reactive Wall“ In Situ Treatment Technology, Nyer, E. K., Fam, S., Kidd, D. F., Johns, F J., Palmer, P. L., Boettcher, G., Crossman, T. L. and Suthersan, S. S., eds., Lewis Publishers, Boca Raton (1996).
Powell, R. M., Puls, R. W, Hightower, S. K. and Sabatini, D. A., “Coupled Iron Corrosion and Chromate Reduction: Mechanisms for Subsurface Remediation,”Environ. Sci. Technol.,29, 1913 (1995).
Puls, R. W, Blowes, D. W. and Gillham, R. W., “Long-Term Performance Monitoring for a Permeable Reactive Barrier at the U.S. Coast Guard Support Center, Elizabeth City, North Carolina,”J. Hazard. Mater,68, 109 (1999a).
Puls, R. W, Paul, C. J. and Powell, R. M., “The Application ofIn Situ Permeable Reactive (Zero-Valent Iiron) Barrier Technology for the Remediation of Chromate-Contaminated Groundwater: a Field Test,”Appl. Geochem.,14, 989 (1999b).
Scherer, M. M., Balko, B. A. and Tratnyek, P. G., “The Role of Oxides in Reduction Reactions at the Metal-Water Interface. In Kinetics and Mechanism of Reactions at the Mineral/Water Interface“ ACS symposium series, Washington DC. (1998).
Shrimali, M. and Singh, K. P., “New Methods of Nitrate Removal from Water,”Environ. Pollution,112, 351 (2001).
Siantar, D. P., Schreier, C. G., Chou, C. S. and Reinhard, M., “Treatment of 1,2-Dibromo-3-Chloropropane and Nitrate-Contaminated Water with Zero-Valent Iron or Hydrogen/Palladium Catalysts,”Wat. Res.,30, 2315 (1996).
Singer, P. C. and Stum, W., “Acid Mine Drainage: Rate-Determining Step,”Science,167, 1121 (1970).
Su, C. and Puls, R. W., “Kinetics of Trichloroethene Reduction by Zero-Valent Iron and Tin: Pretreatment Effect, Apparent Activation Energy, and Intermediate Products,”Environ. Sci. Technol.,33, 163 (1999).
U.S. EPA, “Metal-Enhanced Dechlorination of Volatile Organic Compounds Using an Above-Ground Reactor,” EPA/540/R-96-503. (1997).
U.S. EPA, “Metal-Enhanced Dechlorination of Volatile Organic Compounds Using an In-Situ Reactive Iron Wall,” EPA/540/R-98/501 (1998).
Vogan, J. L., Focht, R M., Clark, D. K. and Graham, S. L., “Performance Evaluation of a Permeable Reactive Barrier for Remediation of Dissolved Chlorinated Solvents in Groundwater,”J. Hazard. Mater,68, 97 (1999).
Weber, E. J., “Iron-Mediated Reductive Transformations: Investigation of Reaction Mechanism,”Environ. Sci Technol.,30, 716 (1996).
Wilson, E. K., “Zero-Valent Metals Provide Possible Solution to Groundwater Problems,”C&EN, July 1,19 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, G., Rho, S. & Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 21, 621–628 (2004). https://doi.org/10.1007/BF02705496
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705496