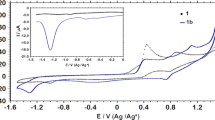
Abstract
This article presents a brief overview of the reactions of2,4,6-tris(2-pyridyl)-1,3,5-triazine (tptz) in presence of rhodium(III), ruthenium(II) and osmium(II) under various experimental conditions. Under certain experimental conditions tptz exhibits metal-assisted hydrolysis/hydroxylation at the triazine ring. However, synthetic methods have also been developed to prepare complexes with intact tptz. Molecular structures of some of the complexes, especially stereoisomers of the hydroxylated products, are established by single crystal X-ray studies. A critical analysis of all data suggests that the electron-withdrawing effect of the metal ion (L→Mσ donation) is the predominant factor, rather than angular strain, that is responsible for metal-promoted reactivities. Electrochemical properties of all of these complexes have been investigated, Rh(III) complexes are excellent catalysts for electrocatalytic reduction of CO2, and dinuclear Ru(II) and Os(II) complexes exhibit strong electronic communication between the metal centres.
Similar content being viewed by others
References
Balzani V, Juris A, Venturi M, Campagna S and Serroni S 1996Chem. Rev. 96 759
Venturi M, Serroni S, Juris A, Campagna S and Balzani V 1998Top. Curr. Chem. 197 193
DeCola L and Belser P 1998Coord. Chem. Rev. 177 301
Balzani V, Campagna S, Denti G, Juris A, Serroni S and Venturi M 1998Acc. Chem. Res. 31 26
Belser P, Bernhard S, Blum C, Beyeler A, DeCola L and Balzani V 1999Coord. Chem. Rev. 190-192 155
Barigelletti F and Flamigni L 2000Chem. Soc. Rev. 29 1
El-Ghayoury A, Harriman A, Khatyr A and Ziessel R 2000Angew. Chem., Int. Ed. Engl. 39 185
Collins P and Diehl H 1960Anal. Chem. Acta 2 125
Diehl H, Buchanan E B Jr. and Smith G F 1960Anal. Chem. 32 1117
Embry W A and Ayres G H 1968Anal. Chem. 40 1499
Janmohamed M J and Ayres G H 1972Anal. Chem. 44 2263
Thomas N C, Foley B L and Rheingold A L 1988Inorg. Chem. 27 3426
Chirayil S, Hegde V, Jahng Y and Thummel R P 1991Inorg. Chem. 30 2821
Berger R M, Ellis II D D 1996Inorg. Chim. Acta 241 1
Paul P, Tyagi B, Bhadbhade M M and Suresh E 1997J. Chem. Soc., Dalton Trans. 2273
Paul P, Tyagi B, Bilakhiya A K, Bhadbhade M M, Suresh E and Ramachandraiah G 1998Inorg. Chem. 37 5733
Paul P, Tyagi B, Bilakhiya A K, Bhadbhade M M and Suresh E 1999J. Chem. Soc., Dalton Trans. 2009
Paul P, Tyagi B, Bilakhiya A K, Dastidar P and Suresh E 2000Inorg. Chem. 39 14
Bilakhiya A K, Tyagi B, Agnihotri P, Suresh E, Dastidar P and Paul P (in press)
Keene F R 1997Coord. Chem. Rev. 166 121
Kelso L S, Reitsma D A, Keene F R 1996Inorg. Chem. 35 5144
Smolin E M, Rapoport L S 1959Triazines and derivatives (New York: Interscience) p. 163
Lerner E I and Lippard S J 1976J. Am. Chem. Soc. 98 5397
Faus J, Julve M, Amigo J M and Debaerdemaeker T 1989J. Chem. Soc., Dalton Trans. 1681
Arana C, Yan S, Keshavart K M, Potts K T and Abruna H D 1992Inorg. Chem. 31 3680
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Paul, P. Ruthenium, osmium and rhodium complexes of polypyridyl ligands: Metal-promoted activities, stereochemical aspects and electrochemical properties. J Chem Sci 114, 269–276 (2002). https://doi.org/10.1007/BF02703819
Issue Date:
DOI: https://doi.org/10.1007/BF02703819