Summary
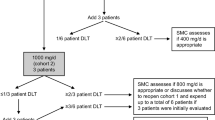
Pentosan polysulfate is a semisynthetic pentasaccharide heparinoid derived from beechwood shavings. A total of nineteen patients with various adult solid tumors were treated with three dose levels (15, 22.5, and 30 mg/m2/dose) of subcutaneous pentosan polysulfate every 6 hours. The dose limiting toxicities were thrombocytopenia and elevated transaminases at the dose of 30 mg/m2 every 6 hours. The recommended starting dose for phase II trials is 22.5 mg/m2 given every 6 hours. There was an increase in anticoagulant activity as measured by activated partial thromboplastin time (aPTT) at the dose of 22.5 mg/m2 every 6 hours in most patients. There were no objective responses and three patients had stable disease lasting 16, 19 and 76 weeks.
Similar content being viewed by others
References
Bergqvist D, Efsing HO, Hallbrook Tet al.: Prevention of postoperative thromboembolic complications. Acta Chir Scand 146: 559–568, 1980
Wagenvoord R, Hendrix H, Soria Cet al.: Localization of the inhibitory site(s) of pentosan polysulfate in blood coagulation. Thrombo and Haemostas 60:220–225, 1988
Wagenvoord R, Hendrix H, Soria Cet al.: Determination of the non-antithrombin III dependent inhibitor sites of pentosan polysulfate in the blood coagulation. First International Symposium on pentosan polysulfate. Paris 1985 (Abstract)
Fischer AM, Merton RE, Marsh NAet al.: A comparison of pentosan polysulfate and heparin II: Effects of subcutaneous injection. Thromb Haemostas 47:109–113, 1982
MacGregor IR, Dawes J, Pepper DSet al.: Metabolism of sodium pentosan polysulfate in man measured by a new competitive binding assay for sulphated polysaccharides—comparison with effects on anticoagulant activity, lipolysis and platelet α-granule proteins. Thromb Haemostas 53: 411–414, 1985
Zugmaier G, Lippman ME, Wellstein A: Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J Natl Can Inst 84: 1716–1724, 1992
Maciag T, Hoover G, Sternerman Met al.: Serial propagation of human endothelial cellsin vitro. J Cell Biol 91:420–426, 1981
Shing Y, Folkman J, Haudenschild Cet al.: Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem 29: 275–287, 1985
Esch F, Baird A, Ling Net al.: Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Nat Acad Sci USA 82: 6507–6511, 1985
Thompson J, Haudenschild C, Anderson Ket al.: Heparin-binding growth factor 1 induces the formation of organoid neovascular structuresin vivo. Proc Nat Acad Sci USA 86: 7928–7932, 1989
Folkman J, Haudenschild C: Angiogenesisin vitro: Nature 288: 551–556, 1980
MacGregor I, Dawes J, Paton Let al.: Metabolism of sodium pentosan polysulfate in man—catabolism of iodinated derivatives. Thromb Haemostas 51:321–325, 1984
Sie P, Albarede J, Robert Met al.: Tolerance and biological activity of pentosan polysulfate after intramuscular or subcutaneous administration for 10 days in human volunteers. Thromb Haemostas 55: 86–89, 1986
Parker BW, Swain SM, Zugmaier Get al.: Detectable inhibition of heparin-binding growth factor activity in sera from patients treated with pentosan polysulfate. J Natl Can Inst 85: 1068–1073, 1993
Ajani JA, Welch SR, Raben MNet al.: Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8: 147–159, 1990
Oken MM, Cruch RH, Tormey DCet al.: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649–655, 1982
Follea G, Hamandjian I, Trzeciak MC,et al.: Pentosan associated thrombocytopenia. Thrombosis Res 42: 413–418, 1986
Pluda JM, Shay LE, Foli Aet al.: Administration of pentosan polysulfate to patients with human immunodeficiency virus-associated Kaposi’s sarcoma. J Natl Can Inst 85: 1585–1592, 1993
Goad KE, Horne MJ, Gralnick HR: Immune mediated thrombocytopenia associated with pentosan polysulfate. Blood 78S: 147a, 1991
Tannenbaum S, Alms L, Goad Ket al.: Pentosan induces thrombocytopenia and platelet dysfunction during continuous IV infusion. Blood 78S: 146a, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Swain, S.M., Parker, B., Wellstein, A. et al. Phase I trial of pentosan polysulfate. Invest New Drugs 13, 55–62 (1995). https://doi.org/10.1007/BF02614221
Issue Date:
DOI: https://doi.org/10.1007/BF02614221