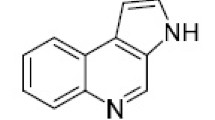
Abstract
Published data on the synthesis of compounds containing the pyrrolo[2,1-a]isoquinoline system in their structure are reviewed. Examples of substances that have useful properties for practical purposes are given.
Similar content being viewed by others
References
G. Ing, Heterocyclic Compounds, R. Elderfield (ed.) [Russian translation], Vol. 3, Izdatinlit, Moscow (1954), p. 310.
T. Uchida and K. Matsumoto, Synthesis, No. 4, 209 (1976).
A. S. Chawla and A. H. Jackson, Natur. Prod. Rept.6, 55 (1989).
S. A. Yunosov, Alkaloids [in Russian], Fan, Tashkent (1981), p. 147.
M. Suffnes and G. A. Cordell, The Alkaloids, A. Brossi (ed.), Vol. 25, Academic Press, New York (1985), p. 57.
M. H. Sarragiotto, H. L. Filho, and A. G. Marsaoli, Can. J. Chem.,59, 2771 (1981).
A. Mondon and P. R. Seidel, Chem. Ber.,104, 2937 (1971).
A. Mondon, Chem. Ber.,104, 2960 (1971).
A. Mondon, A. Gerd, and O. Eckhard, Chem. Ber.,105, 2025 (1972).
A. Mondon, E. Oelrich, and R. Schickfluss, Chem. Ber.,105, 2036 (1972).
A. Mondon, H. G. Vilhuber, C. Fischer, M. Epe, B. Epe, and Ch. Wolff, Chem. Ber.,112, 1110 (1979).
A. Mondon, M. Epe, Ch. Wolff, T. Clauson, and H. G. Vilhuber, Chem. Ber.,112, 1126 (1979).
K. Ito, F. Hiroshi, and H. Mitsumasa, Yakugaku Zasshi,93, 1611 (1973); Chem. Abs.,80, 68387 (1973).
K. Ito, H. Furukawa, and M. Haruna, Yakugaku Zasshi,93, 1617 (1973); Chem. Abs.,80, 48212 (1974).
T. Rasheed, M. N. I. Khan, S. S. A. Zhadi, and S. Durrani, J. Natur. Prod.,54, 582 (1991).
S. Ruchirawat and V. Somchitman, Tetrahedron Lett., No. 46, 4159 (1976).
M. Alvarez, M. Salas, and J. A. Joule, Heterocycles,32, 759 (1991).
W. Loesel, O. Roos, G. Schnorrenberg, D. Arndts, G. Speck, and I. Steller, German Patent No. 3,919,246; Chem. Abs.,114, 183300 (1991).
W. R. Loesel, G. Schnorrenberg, R. Reichl, and H. Ensinger, European Patent No. 190,563; Chem. Abs.,106, 102310 (1987).
W. Loesel, O. Roos, D. Arndts, G. Speck, and J. Streller, German Patent No. 3,918,519; Chem. Abs.,114, 228760 (1991).
Mitsubishi Chem. Ind. Co. Ltd., Japanese Patent No. 57,146,773; Chem. Abs.,98, 143286 (1983).
B. E. Maryanoff, C. A. Maryanoff, D. F. McComsey, and K. L. Sorgi, US Patent No. 4,837,328; Chem. Abs.,112, 20913 (1990).
K. L. Sorgi, C. A. Maryanoff, D. F. McComsey, D. W. Graden, and B. E. Maryanoff, J. Am. Chem. Soc.,112, 3567 (1990).
W. K. Anderson, H. L. McPherson, J. S. New, and A. C. Rick, J. Med. Chem.,27, 1321 (1984).
M. Suehiro, U. Sheffel, R. F. Dannals, H. T. Ravert, G. A. Ricaurte, and H. N. Wagner, Jr., J. Nucl. Med.,34, 120 (1993).
T. Tomita, Y. Inami, and Y. Terada, Chem. Pharm. Bull.,38, 1563 (1990).
M. Suehiro, H. T. Ravert, R. F. Dannals, U. Scheffel, and H. N. Wagner, Jr., J. Labelled Compd. Radiopharm.,31, 841 (1992).
M. Froimowitz, J. Comput. Chem.,14, 934 (1993).
R. P. Shank, J. L. Vaught, K. A. Pelley, P. E. Setler, D. F. McComsey, and B. E. Maryanoff, J. Pharmacol. Exp. Ther.,247, 1032 (1988).
F. A. Chzranowski, B. A. McCrogan, and B. E. Maryanoff, J. Med. Chem.,28, 399 (1985).
Y. Terada, T. Tomita, and Y. Inami, Quant. Struct., Act. Relat.,10, 118 (1991).
D. M. Smith, P. N. Jensen, S. H. Poulsen, E. O. Mikkelsen, E. G. Elbaz, and R. Glaser, Eur. J. Pharmacol.,196, 85 (1991).
M. Suehiro, U. Scheffel, H. T. Ravert, R. F. Dannals, and H. N. Wagner, Jr., Life Sci.,53, 883 (1993).
M. J. Achton, D. I. Dron, G. Fenton, D. J. Lithgoe, C. G. Newton, and D. Riddell, European Patent No. 303,446; Chem. Abs.,112, 178703 (1990).
G. Ferrari and C. Casagrande, British Patent No. 1,153,670; Chem. Abs.,71, 81215 (1969).
W. Loesel, O. Roos, and G. Schnorrenberger, US Patent No. 4,694,085; Chem. Abs.,108, 204513 (1988).
C. Casagrande, A. Invernizzi, R. Ferrini, and G. G. Ferrari, J. Med. Chem.,11, 765 (1968).
H. J. Bertram, R. Fisher, H. Hagemann, B. W. Krueger, T. Shenke, C. Erdelen, B. Krauskopf, K. Luerssen, and H. J. Santel, German Patent No. 4,032,090; Chem. Abs.,115, 232069 (1991).
H. Duerr, H. P. Joensson, P. Spang, T. Muenzmay, and P. Scheihauer, German Patent No. 3,521,432; Chem. Abs.,106, 102089 (1987).
Y. Oguchi, K. Katagiri, and Y. Takasu, Japanese Patent No. 61,108,593; Chem. Abs.,106, 11256 (1987).
K. Katagiri, Y. Oguchi, T. Otake, K. Arao, and Y. Takasu, Japanese Patent No. 6,115,148; Chem. Abs.,105, 52179 (1986).
Mitsubishi Paper Mills Ltd., Japanese Patent No. 60,121,440; Chem. Abs.,103, 224378 (1985).
A. Tanaka, M. Nakatani, and A. Yoshida, Japanese Patent No. 77,120,824; Chem. Abs.,88, 180233 (1978).
V. Boekelheide and J. Godfrey, J. Am. Chem. Soc.,75, 3679 (1953).
R. Child and F. L. Pyman, J. Chem. Soc., No. 1, 36 (1931).
K. Wiesner, Z. Valenta, A. J. Manson, and F. W. Stonner, J. Am. Chem. Soc.,77, 675 (1955).
M. Pailer, W. Brandstetter, Monatsh. Chem.,83, 523 (1952).
J. Bosch, E. Mestre, J. Bonjoch, F. Lopez, and R. Grandos, Heterocycles,22, 767 (1984).
J. B. Bremner and K. N. Winzenberg, Austral. J. Chem.,37, 1203 (1984).
B. E. Maryanoff, D. F. McComsey, J. F. Gardocki, R. P. Shank, M. J. Costanzo, S. O. Schneider, and P. E. Setler, J. Med. Chem.,30, 1433 (1987).
B. E. Maryanoff, D. F. McComsey, and B. A. Duhl-Emswiller, J. Org. Chem.,48, 5062 (1983).
B. E. Maryanoff, D. F. McComsey, H. R. Almond Jr., M. S. Mutter, W. Bemis Guy, R. R. Whittle, and R. Olofson, J. Org. Chem.,51, 1341 (1986).
B. E. Maryanoff and D. F. McComsey, Tetrahedron Lett., No. 40, 3797 (1979).
B. E. Maryanoff, European Patent No. 130,069; Chem. Abs.,103, 87783 (1985).
B. E. Maryanoff, D. F. McComsey, R. R. Inners, M. S. Mutter, G. P. Wooden, S. L. Mayo, and R. A. Olofson, J. Am. Chem. Soc.,111, 8062 (1989).
B. E. Maryanoff, D. F. McComsey, R. R. Inners, G. P. Wooden, S. L. Mayo, and R. A. Olofson, J. Am. Chem. Soc.,111, 2487 (1989).
B. E. Maryanoff and D. F. McComsey, J. Heterocycl. Chem.,22, 911 (1985).
B. E. Maryanoff and H. R. Almond, Jr., J. Org. Chem.,51, 3295 (1986).
B. E. Maryanoff, D. F. McComsey, M. S. Mutter, K. L. Sorgi, and C. A. Maryanoff, Tetrahedron Lett., No. 40, 5073 (1988).
M. Porbez, B. E. Maryanoff, and D. F. McComsey, Acta Crystalogr. Sect. C. Cryst. Struct. Commun.,46, 931 (1990).
R. D. Shah and C. A. Maryanoff, J. Chromatogr., Nos. 1–2, 348 (1991).
R. P. Shank, J. F. Cardocki, C. R. Schneider, J. L. Vaught, P. E. Setler, B. E. Maryanoff, and D. F. McComsey, J. Pharmacol. Exp. Ther.,242, 74 (1987).
B. E. Maryanoff, D. F. McComsey, M. J. Constanzo, P. E. Setler, J. F. Gardocki, R. P. Shank, and C. R. Schneider, J. Med. Chem.,27, 943 (1984).
M. Winn and H. E. Zaugg, J. Org. Chem.,33, 3779 (1968).
J. P. Freeman and M. K. Fettes-Fields, Heterocycles,23, 1073 (1985).
H. End, H. De Koning, and W. N. Speckamp, J. Org. Chem.,51, 1687 (1986).
J. Dijkink and W. N. Speckamp, Tetrahedron Lett., No. 11, 935 (1977).
E. Lete, A. Egiarte, N. Sotomayor, T. Vicente, and M. J. Villa, Synlett., No. 1, 41 (1993).
K. D. Moeller, W. Wang Po, S. Tarazi, R. Marzabadi Mohammad, and P. L. Wong, J. Org. Chem.,56, 1058 (1991).
T. Shono, Y. Matsumura, and K. Tsubata, J. Am. Chem. Soc.,103, 1172 (1981).
H. H. Wasserman, R. Frechette, T. Oida, and J. H. Van Duzer, J. Org. Chem.,54, 6012 (1989).
H. H. Wasserman, J. Fukujama, N. Murugesan, J. Duzer, L. Lombardo, V. Rotello, and K. McCarthy, J. Am. Chem. Soc.,111, 371 (1989).
W. K. Anderson and R. K. Frederick, Jr., J. Heterocycl. Chem.,27, 975 (1990).
F. Morlacchi and V. Losacco, J. Heterocycl. Chem.,13, 165 (1976).
F. M. Shell and A. M. Smith, Tetrahedron Lett., No. 18, 1883 (1983).
H. W. Gibson, J. Heterocycl. Chem.,26, 361 (1989).
B. C. Uff, R. S. Budhram, M. F. Consterdine, and J. K. Hicks, J. Chem. Soc. Perkin Trans. I, No. 18, 2018 (1977).
G. Schmitt, D. An. Nguen, B. Laude, and J. Vebrel, Bull. Soc. Chim. Belg.,96, 535 (1987).
J. Kant, F. D. Popp, and B. C. Uff, J. Heterocycl. Chem.,22, 1065 (1985).
G. Schmitt, B. Laude, J. Vebrel, N. Rodier, and F. Theobald, Bull. Soc. Chim. Belg.,98, 113 (1989).
W. E. McEwen, C. C. Cabello, M. A. Calabro, A. M. Ortega, P. E. Stott, and A. J. Zapata, J. Org. Chem.,44, 111 (1979).
W. E. McEwen, I. C. Mineo, and Y. H. Shen, J. Am. Chem. Soc.,93, 4481 (1971).
J. T. Hahn, J. Kant, F. D. Popp, S. R. Chabra, and B. C. Uff, J. Heterocycl. Chem.,29, 1165 (1992).
W. E. McEwen, I. C. Wang Huang, C. P. Cartaya Marin, F. McCarthy, E. M. Segnini, Ch. M. Zepp, and J. J. Lubinkowski, J. Org. Chem.,47, 3098 (1982).
R. Huisgen, R. Grashey, and E. Steingruber, Tetrahedron Lett., No. 22, 1441 (1963).
T. Kutsuma, Y. Sekine, K. Fujijama, and Y. Kobayashi, Chem. Pharm. Bull.,20, 2701 (1972).
C. A. Henrick, E. Ritchie, and W. C. Taylor, Austral. J. Chem.,20, 2467 (1967).
Y. Kobayashi, I. Kumadaki, Y. Sekine, and T. Kutsuma, Chem. Pharm. Bull.,21, 1118 (1973).
M. Kiyoshi, I. Yukio, K. Hideyuki, S. Xiao-Ian, U. Takane, and A. Kinuyo, J. Heterocycl. Chem.,25, 689 (1988).
T. Sasaki, K. Kanematsu, and Y. Yukimoto, J. Chem. Soc., No. 3, 481 (1970).
T. Kato, T. Chiba, and H. Kimura, Chem. Pharm. Bull.,25, 203 (1977).
J. Stetinova, M. Dandarova, J. Kovac, J. Lesko, F. Povazanec, and A. Pajchortova, Czechoslovakian Patent No.230,333; Chem. Abs.,108, 221603 (1988).
J. Stetinova, J. Kovac, F. Povazanec, M. Dandarova, and A. Pajchortova, Collect. Czech. Chem. Commun.,49, 533 (1984).
R. M. Acheson and P. J. Ansell, J. Chem. Soc. Perkin Trans I, No. 6, 1275 (1987).
S. Kanemasa, S. Takenaka, H. Watanabe, and O. Tsuge, J. Org. Chem.,54, 420 (1989).
J. Fröhlich and F. Kröhnke, Chem. Ber.,104, 1621 (1971).
Y. Tominaga, Y. Ichinara, T. Mori, C. Kamio, and A. Hosomi, J. Heterocycl. Chem.,27, 263 (1990).
N. S. Basketter and A. O. Plunkett, J. Chem. Soc. Chem. Commun., No. 1, 188 (1973).
O. Tsuge, S. Kanemasa, K. Sakamoto, and S. Takenaka, Bull. Chem. Soc. Jpn.,61, 2513 (1988).
Y. Tominaga, Y. Shiroshita, T. Kurokawa, H. Gotou, Y. Matsuda, A. Hosomi, J. Heterocycl. Chem.,26, 477 (1989).
H. Fujito, Y. Tominaga, Y. Matsuda, and G. Kobayashi, Yakugaku Zasshi,97, 1316 (1977); Chem. Abs.,88, 152384 (1989).
O. Tsuge, S. Kanemasa, and S. Takenaka, Chem. Lett., No. 3, 355 (1985).
O. Tsuge, S. Kanemasa, and S. Takenaka, Bull. Chem. Soc. Jpn.,58, 3320 (1985).
O. Tsuge, S. Kanemasa, and S. Takenaka, Bull. Chem. Soc. Jpn.,59, 3631 (1986).
O. Tsuge, S. Kanemasa, and S. Takenaka, Bull. Chem. Soc. Jpn.,58, 3137 (1985).
Y. Tominaga, S. Hidaki, Y. Matsuda, G. Kobayashi, and K. Sakemi, Yakugaku Zasshi,99, 540 (1979); Chem. Abs.,91, 157559 (1979).
K. Matsumoto, Y. Ikemi-Kono, T. Uchida, and L. A. Paquette, Fukusokan Kagaku Toronkai Koen Yoshishu, No. 13, 101 (1979); Chem. Abs.,93, 46381 (1980).
Yu. A. Sharanin, V. N. Nesterov, L. A. Rodinovskaya, V. E. Shklover, Yu. T. Struchkov, and V. P. Litvinov, Khim. Geterotsikl. Soedin., No. 9, 1248 (1991).
A. F. Khlebnikov, E. I. Kostik, R. R. Kostikov, and V. Ya. Bespalov, Khim. Geterotsikl. Soedin., No. 3, 355 (1991).
T. Kato, T. Chipa, and T. Sasaki, Heterocycles,12, 925 (1979).
H. Fujito, Y. Tominaga, H. Awaya, Y. Matsuda, and G. Kobayashi, Yakugaku Zasshi,98, 1412 (1978); Chem. Abs.,90, 72024 (1979).
A. M. Shestopalov, V. P. Litvinov, Yu. A. Sharanin, and G. E. Khoroshilov, Dokl. Akad. Nauk,312, 1156 (1990).
A. M. Shestopalov, L. A. Rodinovskaya, Yu. A. Sharanin, and V. P. Litvinov, Izv. Akad. Nauk SSSR, Ser. Khim., No. 11, 2593 (1990).
L. Weber, Magu. Kem. Lapja,40, 74 (1985); Chem. Abs.,105, 42717 (1986).
T. Tischer, L. Toke, and G. Toth, Acta Chim. Hung.,127, 171 (1990).
R. Grigg and F. Heaney, J. Chem. Soc. Perkin Trans. I, No. 1, 198 (1989).
Z. Bende, L. Toke, L. Weber, G. Toth, F. Janke, and G. Csonka, Tetrahedron,40, 369 (1984).
G. Toth, J. Frank, Z. Bende, L. Weber, and K. Simou, J. Chem. Soc. Perkin Trans. I, No. 9, 1961 (1983).
T. Kato, T. Chiba, and T. Sasaki, Yakugaku Zasshi,99, 1051 (1979); Chem. Abs.,92, 163827 (1980).
T. Kato, T. Chiba, S. Tanaka, and T. Sasaki, Chem. Pharm. Bull.,25, 2697 (1977).
Y. Yamashita, Y. Miyauchi, and M. Masumura, Chem. Lett., No. 4, 489 (1983).
M. Sato, N. Kanuma, and T. Kato, Chem. Pharm. Bull.,30, 4359 (1982).
M. Sato, N. Kanuma, and T. Kato, Chem. Pharm. Bull.,32, 106 (1984).
K. Matsumoto and T. Uchida, Heterocycles,12, 661 (1979).
K. Matsumoto and T. Uchida, J. Chem. Soc. Perkin Trans. I, No. 1, 73 (1981).
J. Alvarez-Builla, M. Gloria Quintanilla, C. Abril, and M. T. Gandasegui, J. Chem. Res. Synop., No. 6, 202 (1984).
R. Huisgen and K. Niklas, Heterocycles,22, 21 (1984).
K. Mizuyama, Y. Matsuo, Y. Tominaga, Y. Matsuda, and G. Kobayashi, Chem. Pharm. Bull.,24, 1299 (1976).
Y. Tominaga, H. Gotou, Y. Oniyama, Y. Nishimura, and Y. Matsuda, Chem. Pharm. Bull.,33, 3038 (1985).
B. Hershenson, J. Org. Chem.,40, 740 (1975).
R. Grigg, N. N. Q. Gunarathe, D. Henderson, and V. Sridharan, Tetrahedron,46, 1599 (1990).
R. Grigg, P. Myers, A. Somasunderam, and V. Sridharan, Tetrahedron,48, 9735 (1992).
R. Grigg, P. Kennewell, V. Savic, and V. Sridharan, Tetrahedron,48, 1023 (1992).
R. Huisgen and H. Seidl, Tetrahedron Lett., No. 29, 2019 (1963).
R. Huisgen, H. Hauck, R. Grashey, and H. Seidl, Chem. Ber.,101, 2568 (1968).
S. Kato, T. Yokomatsu, Y. Yuasa, and S. Shibuya, Heterocycles,19, 2143 (1982).
Y. Kobayashi, I. Kumadaki, and S. Fujino, Heterocycles,7, 871 (1977).
E. Breuer, S. Zbaida, and J. Pesso, Tetrahedron,33, 1145 (1977).
M. Burdisso, A. Gamba, and R. Gandolfi, Tetrahedron,44, 3735 (1988).
R. M. Acheson, Advances in Heterocyclic Chemistry, New York, London: Academic Press,1, 125 (1963).
R. H. Wiley and L. H. Knabeschuh, J. Org. Chem.,18, 836 (1953).
R. M. Acheson and F. Hole, J. Chem. Soc., No. 3, 748 (1962).
R. M. Acheson and M. S. Verlander, J. Chem. Soc., No. 17, 2311 (1969).
J. W. Lown and K. Matsumoto, Can. J. Chem.,49, 1165 (1971).
J. W. Lown and K. Matsumoto, Can. J. Chem.,49, 3119 (1971).
T. Eicher and D. Krause, Tetrahedron Lett., No. 14, 1213 (1979).
T. Eicher and W. Freihoff, Synthesis, No. 11, 908 (1986).
K. B. Vatsuro and G. A. Mishchenko, Named Reactions in Organic Chemistry [in Russian], Moscow, Khimiya (1976).
R. H. Sprague, US Patent No. 2,622,082; Chem. Abs.,47, 3159 (1953).
D. Danhardt and R. Obergrusberber, Arch. Pharm.,312, 896 (1979).
M. D. Nair and J. A. Desai, Indian J. Chem. Sect. B,19B, 65 (1980).
Y. Ban and M. Terashima, Tetrahedron Lett., No. 22, 796 (1961).
Y. Ban and M. Terashima, Chem. Pharm. Bull.,13, 775 (1965).
A. Buzas, J. Merour, and G. Lavielle, Heterocycles,23, 2561 (1985).
A. Buzas and G. Lavielle, German Patent No. 3,027,325; Chem. Abs.,95, 25378 (1981).
K. Nagarajan, P. J. Rodrigues, and M. Netraju, Tetrahedron Lett., No. 47, 7229 (1992).
H. Heyer, Annalen, No. 9, 1534 (1981).
Y. Akiyama, J. Abe, T. Takano, T. Kawasaki, and M. Sakamoto, Chem. Pharm. Bull.,32, 2821 (1984).
S. G. Agbalyan and L. A. Nersesyan, Arm. Khim. Zh.,22, 719 (1969).
S. G. Agbalyan and L. A. Nersesyan, Arm. Khim. Zh.,20, 447 (1967).
S. G. Agbalyan, R. D. Khachikyan, and K. K. Lulukyan, Khim. Geterotsikl. Soedin., No. 7, 943 (1979).
S. G. Agbalyan, K. K. Lulukyan, and G. V. Grigoryan, Enamines in Organic Synthesis [in Russian], Ur. Otd. Akad. Nauk SSSR, Sverdlovsk (1989), p. 60.
M. Sakamoto, T. Akimoto, Y. Akiyama, K. Fukutomi, and K. Ishii, Chem. Pharm. Bull.,32, 1170 (1984).
T. Naito, O. Miyata, and I. Ninomiya, J. Chem. Soc. Chem. Commun., No. 11, 517 (1979).
V. S. Shklyaev, B. B. Aleksandrov, G. I. Legotkina, M. I. Vakhrin, M. S. Gavrilov, and A. G. Mikhailovskii, Khim. Geterotsikl. Soedin., No. 11, 1560 (1983).
M. Yu. Dormidontov, B. Ya. Syropyatov, R. Z. Dautova, B. B. Aleksandrov, V. S. Shklyaev, M. I. Vakhrin, and A. G. Mikhailovskii, Khim. Farm. Zh., No. 1, 22 (1990).
B. B. Aleksandrov, M. S. Gavrilov, M. I. Vakhrin, and V. S. Shklyaev, Khim. Geterotsikl. Soedin., No. 6, 794 (1985).
V. S. Shklyaev, B. B. Aleksandrov, A. G. Mikhailovskii, and M. I. Vakhrin, Khim. Geterotsikl. Soedin., No. 9, 1239 (1989).
B. B. Aleksandrov, V. S. Shklyaev, and Yu. V. Shklyaev, Khim. Geterotsikl. Soedin., No. 3, 375 (1992).
V. D. Sviridov, N. D. Chkanikov, M. V. Galakhov, Yu. V. Shklyaev, V. S. Shklyaev, and B. B. Aleksandrov, and M. S. Gavrilov, Izv. Akad. Nauk SSSR. Ser. Khim., No. 6, 1405 (1990).
V. D. Sviridov, N. D. Chkanikov, Yu. V. Shklyaev, A. F. Kolomiets, and A. V. Fokin, Khim. Geterotsikl. Soedin., No. 12, 1689 (1990).
T. Sano, J. Toda, N. Kashiwaba, Y. Tsuda, and Y. Iitaka, Heterocycles,16, 1151 (1981).
T. Sano, J. Synth. Org. Chem. Jpn.,42, 340 (1984).
C. Saã, E. Guitiãn, and L. Castedo, J. Org. Chem.,51, 2781 (1986).
V. S. Shklyaev, B. B. Aleksandrov, A. G. Mikhailovskii, and M. I. Vakhrin, Khim. Geterotsikl. Soedin., No. 7, 963 (1987).
L. Castedo, C. Saã, L. M. Saã, and R. Suau, J. Org. Chem.,47, 513 (1982).
S. V. Vessar, P. Singh, and S. K. Sharma, Tetrahedron Lett., No. 40, 4179 (1982).
B. B. Aleksandrov, V. S. Shklyaev, and Yu. V. Shklyaev, Khim. Geterotsikl. Soedin., No. 6, 854 (1991).
A. G. Mikhailovskii, V. S. Shklyaev, and B. B. Aleksandrov, Khim. Geterotsikl. Soedin., No. 6, 808 (1990).
A. G. Mikhailovskii and V. S. Shklyaev, Khim. Geterotsikl. Soedin., No. 7, 946 (1994).
A. G. Mikhailovskii, V. S. Shklyaev, G. A. Veikhman, and M. I. Vakhrin, Khim. Geterotsikl. Soedin., No. 10, 1374 (1993).
A. G. Mikhailovskii, Khim. Geterotsikl. Soedin., No. 5, 685 (1996).
A. G. Mikhailovskii and V. S. Shklyaev, Khim. Geterotsikl. Soedin., No. 5, 650 (1995).
Yu. N. Bubnov, A. Yu. Zykov, A. V. Ignatenko, A. G. Mikhailovskii, Yu. V. Shklyaev, and V. S. Shklyaev, Izv. Russk. Akad. Nauk. Ser. Khim., No. 4, 935 (1996).
K. Orito, T. Matsuzaki, H. Suginome, and R. Rodrigo, Heterocycles,27, 2403 (1988).
S. G. Agbalyan and L. A. Nersesyan, Izv. Akad. Nauk Arm. SSR. Khim. Nauk,17, 441 (1964).
V. Carelli, M. Cardellini, F. Liberatore, and F. Morlacchi, Ann. Chim.,51, 477 (1961).
S. F. Dyke, P. A. Bather, and A. B. Garry, Tetrahedron,29, 3881 (1973).
G. Dörnyei and Cs. Szántay, Acta Chimica Academ. Scient. Hung.,89, 161 (1976).
R. Cahill and T. A. Crabb, Organic Magnetic Resonance,4, 259 (1972).
W. Lösel and H. Daniel, Chem. Ber.,118, 413 (1985).
W. Loesel, O. Koos, and G. Schnorrenberg, German Patent No. 3,401,018; Chem. Abs.,104, 5793 (1986).
W. Lösel, Chem. Ber.,121, 547 (1988).
K. Matoba, Y. Miyata, and T. Jamazaki, Chem. Pharm. Bull.,31, 476 (1983).
T. Shono, H. Hamaguchi, M. Sasaki, S. Fujita, and K. Nagami, J. Org. Chem.,48, 1621 (1983).
N. Nakayama, N. Imanura, T. Kanazawa, S. Yoneda, T. Sugimoto, and Z. Yoshida, Heterocycles,20, 168 (1983).
H. McNab and L. C. Monahan, J. Chem. Soc. Chem. Commun., No. 3, 138 (1987).
W. Kiel and F. Kröhnke, Chem. Ber.,105, 3709 (1972).
S. Kano, Y. Yuasa, and S. Shibuya, Synth. Commun.,15, 883 (1985).
A. I. Meyers, J. Guiles, J. S. Warmus, and M. A. Gonzales, Tetrahedron Lett., No. 40, 5505 (1991).
A. I. Meyers and J. Guiles, Tetrahedron Lett., No. 20, 2813 (1990).
S. J. Danishefsky and J. S. Panek, J. Am. Chem. Soc.,109, 917 (1987).
K. Giller, M. S. Baird, and A. De Meijere, Synlett., No. 6, 524 (1992).
Y. Yamawaki, M. Watanabe, S. Yamamura and S. Saito, Yakugaku Zasshi,97, 127 (1977); Chem. Abs.,87, 23003 (1977).
J. C. Hubert, W. Steege, and W. N. Speckamp, Synth. Commun.,1, 103 (1971).
R. Yamaguchi, K. Mochizuki, S. Kozima, and H. Takaya, Chem. Lett., No. 10, 1809 (1994).
S. Saito, T. Tanaka, K. Kotera, H. Nakai, and N. Sugimoto, Chem. Pharm. Bull.,13, 786 (1965).
K. T. Potts and S. Yao, J. Org. Chem.,44, 977 (1979).
S. F. Dyke and R. G. Kinsman, Chemistry of Heterocyclic Compounds, A. Weissberger (ed.), Wiley Interscience, Vol. 38, New York, London (1981), p. 112.
A. Cobas, E. Guitiãn, L. Castedo, and J. M. Saã, Tetrahedron Lett., No. 20, 2491 (1988).
A. Cobas, E. Guitiãn, and L. Castedo, J. Org. Chem.,57, 6765 (1992).
T. Sano, J. Toda, and Y. Tsuda, Chem. Pharm. Bull.31, 356 (1983).
Y. Tsuda, T. Ohshima, T. Sano, and J. Toda, Heterocycles,19, 2027 (1982).
Y. Tsuda, T. Ohshima, T. Sano, and J. Toda, Heterocycles,19, 2053 (1982).
T. Sano, J. Toda, N. Kashiwaba, T. Ohshima, and Y. Tsuda, Chem. Pharm. Bull.,35, 479 (1987).
T. Sano, J. Toda, N. Maehara, and Y. Tsuda, Can. J. Chem.,65, 94 (1987).
T. Sano, J. Toda, T. Ohshima, and Y. Tsuda, Chem. Pharm. Bull.,40, 873 (1992).
T. Sano, J. Toda, Y. Tsuda, and T. Ohshima, Heterocycles,22, 49 (1984).
T. Sano, J. Toda, Y. Horiguchi, K. Imafuku, and Y. Tsuda, Heterocycles,16, 1463 (1981).
T. Sano, J. Toda, and Y. Tsuda, Heterocycles,22, 53 (1984).
T. Sano and Y. Tsuda, J. Synth. Org. Chem. Jpn.,46, 49 (1988).
A. G. Mikhailovskii, V. S. Shklyaev, A. V. Ignatenko, and M. I. Vakhrin, Khim. Geterotsikl. Soedin., No. 7, 934 (1995).
A. G. Mikhailovskii and V. S. Shklyaev, Khim. Geterotsikl. Soedin., No. 12, 1697 (1995).
J. B. Bremner and K. N. Winzenberg, Chem. Ind., No. 10, 421 (1980).
J. B. Bremner and K. N. Winzenberg, Chem. Ind., No. 9, 319 (1979).
J. B. Bremner and Ch. Drager, Heterocycles,23, 1451 (1985).
H. Irikawa, S. Doe, and Y. Okumura, Bull. Chem. Soc. Jpn.,61, 3365 (1988).
D. A. Burnett and D. J. Hart, J. Org. Chem.,52, 5662 (1987).
M. P. Wentland, Tetrahedron Lett., No. 12, 1477 (1989).
H. H. Wasserman and R. M. Amici, J. Org. Chem.,54, 5843 (1989).
V. Prelog, A. Langemann, O. Rodig, M. Ternbah, Helv. Chim. Acta,42, 1301 (1959).
J. P. Yardley, R. W. Rees, and H. Smith, J. Med. Chem.,10, 1088 (1967).
G. P. Shkil', V. I. Terenin, E. L. Dordina, E. G. Atavin, Yu. G. Bundel', and R. S. Sagitullin, Khim. Geterotsikl. Soedin., No. 3, 421 (1987).
Additional information
Institute of Technical Chemistry, Urals Branch, Russian Academy of Sciences, Perm'. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 3, pp. 291–317, March, 1997.
Rights and permissions
About this article
Cite this article
Mikhailovskii, A.G., Shklyaev, V.S. Pyrrolo[2,1-a]isoquinolines (review). Chem Heterocycl Compd 33, 243–265 (1997). https://doi.org/10.1007/BF02253103
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02253103