Abstract
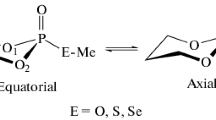
The empirical (MM2) and semiempirical (AM1, MNDO) methods were used to calculate the energy of unsubstituted and 2-, 3-, 5-, 3,5-, and 2,3,5-substituted 1,3,2-oxazaborinanes with complete geometrical optimization. The major minimum on the potential energy surface corresponds to the sofa conformation. The number of other local minima relates to the half-chair and 1,4-, 3,6-, and 2,5-twist conformations. The experimental ΔG0 value for 5-methyl group on the C(5) carbon atom was determined by comparisom of calculated and experimental spin-spin coupling constants.
Similar content being viewed by others
References
A. I. Gren' and V. V. Kuznetsov,Chemistry of Cyclic Borate Esters [in Russian], Naukova Dumka, Kiev (1988).
A. R. Kalyuskii, Author's Abstract of Chemical Sciences Candidate's Dissertation, Odessa (1990).
A. R. Kalyuskii, V. V. Kuznetsov, Yu. E. Shapiro, S. A. Bochkor, and A. I. Gren',Khim. Geterotsikl. Soedin., No. 10, 1424 (1990).
A. R. Kalyuskii, V. V. Kuznetsov, O. S. Timofeev, and A. I. Gren',Zh. Obshch. Khim.,60, 2093 (1990).
A. R. Kalyuskii, V. V. Kuznetsov, Yu. É. Brusilovskii, V. Ya. Gorbatyuk, M. G. Glukhova, and A. I. Gren',Zh. Org. Khim.,26, 2498 (1990).
A. R. Kalyuskii, V. V. Kuznetsov, and A. I. Gren',Zh. Obshch. Khim.,61, 1351 (1991).
A. R. Kalyuskii, V. V. Kuznetsov, N. E. Kruglyak, I. V. Yudanova, L. P. Trigub, and A. I. Gren]Dep. v VINITI, No. 1113-V90; Ref. Zh. Khim., No. 11, B1095 (1990).
R. C. Bingham, M. J. S. Dewar, and D. H. Lo,J. Am. Chem. Soc.,97, 1294 (1975).
P. Birner and H. Hofmann,Int. J. Quant. Chem.,21, 833 (1982).
N. L. Allinger,J. Am. Chem. Soc.,99, 8127 (1977).
M. J. S. Dewar, E. G. Zoebisch, E. P. Healy, and J. J. P. Stewart,J. Am. Chem. Soc.,107, 3902 (1985).
M. J. S. Dewar, C. Jie, and E. C. Zoebisch,Organometallics,7, 53 (1988).
M. J. S. Dewar and W. Thiel,J. Am. Chem. Soc.,99, 4899 (1977).
M. J. S. Dewar and M. L. McKee,J. Am. Chem. Soc.,99, 5231 (1977).
S. Kuribayashi,Bull. Soc. Chem. Japan,46, 1045 (1973).
R. Seip and H. Seip,J. Mol. Struct.,28, 441 (1975).
V. V. Kuznetsov, A. R. Kalyuskii, and A. I. Gren',Khim. Geterotsikl. Soedin., No. 1, 106 (1996).
P. L. Durette and D. Horton,Org. Magn. Reson.,3, 417 (1971).
M. L. Huggins,J. Am. Chem. Soc.,75, 4123 (1953).
N. S. Zefirov, V. S. Blagoveshchenskii, I. V. Kazimirchik, and O. P. Yakovleva,Zh. Org. Khim.,7, 594 (1971).
Additional information
A. V. Bogatskii Institute of Physical Chemistry, National Academy of Sciences of Ukraine, 270080 Odessa, Ukraine. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1058–1064, August, 1999.
Rights and permissions
About this article
Cite this article
Kuznetsov, V.V. Conformational analysis of substituted 1,3,2-oxazaborinanes. Chem Heterocycl Compd 35, 928–934 (1999). https://doi.org/10.1007/BF02252160
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02252160