Abstract
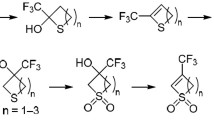
The use of hydrogen sulfide at the moment of formation is proposed for the recyclization of furans into thiophenes in acidic media. The advantages of this method are shown.
Similar content being viewed by others
References
V. G. Kharchenko, I. A. Markushina, and T. I. Gubina,Dokl. Akad. Nauk SSSR,255, 1144 (1980).
V. G. Kharchenko, T. I. Gubina, and I. A. Markushina,Zh. Org. Khim.,18, No. 2, 394 (1982).
V. G. Kharchenko, T. I. Gubina, S. P. Voronin, and I. A. Markushina,Khim. Geterotsikl. Soedin., No. 11, 1144 (1986).
A. Lupi and B. Chubar,Salt Effects in Organic and Organometallic Chemistry [Russian translation], Mir, Moscow.
H. D. Hartough,Thiophene and Its Derivatives, Interscience, New York (1952).
Additional information
N. G. Chernyshevskii Saratov State University, Saratov 410026, Russia. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 736–738, June, 1999.
Rights and permissions
About this article
Cite this article
Gubina, T.I., Drevko, B.I., Fedina, L.N. et al. New method for synthesis of 2,5-disubstituted thiophenes. Chem Heterocycl Compd 35, 650–652 (1999). https://doi.org/10.1007/BF02251619
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02251619