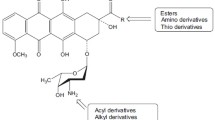
Abstract
A review of physicochemical and analytical properties of anthracycline antitumour agents is presented. The following subjects are discussed: protolytic equilibria, partition and partition coefficients, self-association, adsorptive properties, metal complexation, spectroscopy and chromatography. Furthermore, the stability of anthracyclines in solutions, in pharmaceutical preparations and in biological media is discussed.
Similar content being viewed by others
References
Arcamone F, Franceschi G, Orezzi P, Cassinelli G, Barbieri W, Mondelli R. Daunomycin. I. The structure of daunomycinone. J Am Chem Soc 1964:86:5334–5.
Arcamone F, Cassinelli G, Orezzi P, Franceschi G, Mondelli R. Daunomycin. II. The structure and stereo-chemistry of daunosamine. J Am Chem Soc 1964; 86:5335–6.
Arcamone F, Franceschi G, Penco S, Selva A. Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Lett 1969:13:1007–10.
Arcamone F, Cassinelli G, Franceschi G, Orezzi P, Mondelli R. The total absolute configuration of daunomycin. Tetrahedron Lett 1968:30:3353–6.
Nakata Y, Hopfinger AJ. An extended conformational analysis of doxorubicin. FEBS Lett 1980; 117:259–64.
Arcamone F. Doxorubicin. Anticancer antibiotics. New York: Academic Press, 1981.
Pratt WB, Ruddon RW. The anticancer drugs. New York-Oxford: Oxford University Press, 1979:155–70.
Dorr RT, Fritz WL. Cancer chemotherapy handbook. London: Elsevier Publishing Co., 1980:373–8, 388–401.
Myers CE. Anthracyclines. In: Chabner B, ed. Pharmacological principles of cancer treatment. Philadelphia: WB Saunders Company, 1982:416–34.
Myers CE. Anthracyclines. In: Pinedo HM, Chabner BA, eds. The Cancer Chemotherapy Annual 6. Amsterdam: Elsevier Science Publishers BV, 1984:58–84. (The EORTC Cancer Chemotherapy Annual.)
Brown JR. Adriamycin and related anthracycline antibiotics. Progr Med Chem 1978;15:126–64.
Brown JR, Imam SH. Recent studies on doxorubicin and its analogues. Progr Med Chem 1984;24:170–236.
Montali U, Del Tacca M, Bernardini C, Segnini D, Solaini G. Cardiotoxic effects of adriamycin and mitochondrial oxidation in rat cardiac tissue. Drugs Exp Clin Res 1985;11:219–22.
Carter SK. Adriamycin — a review. J Natl Cancer Inst 1975;55:1265–73.
Wilkinson PM. Clinical pharmacology of adriamycin. In: Pinedo HM, ed. Clinical Pharmacology of Antineoplastic Drugs. Vol. 1. Amsterdam-New York: Elsevier North Holland/Biomedical Press, 1978:209–23.
Davis HL, Davis TE. Daunorubicin and adriamycin in cancer treatment: an analysis of their roles and limitations. Cancer Treat Rep 1979:63:809–15.
DiMarco A. Anthracyclines in cancer chemotherapy. Drugs Exp Clin Res 1983;9:751–65.
Aubel-Sadron G, Londos-Gagliardi D. Daunorubicin and doxorubicin, anthracycline antibiotics, a physico-chemical and biological review. Biochimie 1984;66:333–52.
Blum RH. An overview of studies with adriamycin (NSC-123127) in the United States. Cancer Chemother Rep (Part 3) 1975;6:247–51.
Crooke ST, Reich SD. Anthracyclines: current status and new developments. New York: Academic Press, 1980.
Villani F, Beretta G, Guindani A. Evaluation of early doxorubicin-induced cardiotoxicity by means of systolic time intervals. Cancer Chemother Pharmacol 1979; 3:249–51.
Benjamin RS, Mason YW, Billingham ME. Cardiac toxicity of adriamycin-DNA complex and rubidazone: evaluation by electrocardiogram and endomyocardial biopsy. Cancer Treat Rep 1978;62:935–9.
Tong GL, Henry DW, Acton EM. 5-Iminodaunorubicin. Reduced cardiotoxic properties in an antitumor anthracycline. J Med Chem 1978;22:36–9.
Jaenke RS, Deprez-DeCampeneere D, Trouet A. Cardiotoxicity and comparative pharmacokinetics of six anthracyclines in the rabbit. Cancer Res 1980;40: 3530–6.
Kirchner E, Hartl A, Guttner J, Fritsch S, Hoffmann H. Comparative studies on cardiotoxicity of some anthracycline antitumor antibiotics. Arch Toxicol [Suppl] 1980;4:399–401.
Afanas'ev IB, Polozova NI, Samokhvalov GI. Investigation of the interaction of Superoxide ion with adriamycin and the possible origin of cardiotoxicity of the anthracycline anticancer antibiotics. Bioorg Chem 1980;9:434–9.
Olson RD, Boerth RC, Gerber JG, Nies AS. Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci 1981:29:1393–401.
Lown JW, Chen HH, Plambeck JA, Acton EM. Further studies on the generation of reactive oxygen species from activated anthracyclines and the relationship to cytotoxic action and cardiotoxic effects. Biochem Pharmacol 1982;31:575–81.
Fabregat I, Satrustegui J, Machado A. Interaction with protein SH groups could be involved in adriamycin cardiotoxicity. Biochem Med 1984:32:289–95.
Barbieri B, Bellini O, Savi G, Bertazzoli C, Penco S, Casazza AM. Antitumour activity and cardiotoxicity of new 4-demethoxyanalogues of daunorubicin and doxorubicin. Drugs Exp Clin Res 1984;10:85–90.
Jensen RA, Acton EM, Peters JH. Doxorubicin cardiotoxicity in the rat: comparison of electrocardiogram, transmembrane potential, and structural effects. J Cardiovasc Pharmacol 1984;6:186–200.
Unverferth DV, Leier CV, Balcerzak SP, Hamlin RL. Usefulness of a free radical scavenger in preventing doxorubicin-induced heart failure in dogs. Am J Cardiol 1985;56:157–61.
Garnick MB, Weiss GR, Steele GD, et al. Clinical evaluation of long-term, continuous-infusion of doxorubicin. Cancer Treat Rep 1983:67:133–42.
Weiss AJ, Manthel RW. Experience with the use of adriamycin in combination with other anticancer agents using a weekly schedule, with particular reference to lack of cardiac toxicity. Cancer 1977;40:2046–52.
Brenner DE, Grosh WW, Noone R, Stein R, Greco FA, Hande KR. Human plasma pharmacokinetics of doxorubicin: comparison of bolus and infusional administration. Cancer Treat Symp 1984;3:77–83.
Lokich J, Bothe A, Zipoli T, et al. Constant infusion schedule for adriamycin: A phase I–II clinical trial of a 30-day schedule by ambulatory pump delivery system. J Clin Oncol 1983;1:133–42.
Weiss AJ, Metter GE, Fletcher WS, Wilson WL, Grage TB, Ramirez G. Studies on adriamycin using a weekly regimen demonstrating its clinical effectiveness and lack of cardiac toxicity. Cancer Treat Rep 1976;60:813–22.
Lum BL, Svec JM, Torti FM. Doxorubicin: alteration of dose scheduling as a means of reducing cardiotoxicity. Drug Intell Clin Pharm 1985; 19:259–64.
Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 1982;96:133–9.
Mimnaugh EG, Siddik ZH, Drew R, Sikic BI, Gram TE. The effects of alpha-tocopherol on the toxicity, disposition, and metabolism of adriamycin in man. Toxicol Appl Pharmacol 1979;49:119–26.
Mimnaugh EG, Gram TE, Trush MA. Stimulation of mouse heart and liver microsomal lipid peroxidation by anthracycline anticancer drugs: characterization and effects of reactive oxygen scavengers. J Pharmacol Exp Ther 1983;226:806–16.
Myers CE, McGuire WP, Young RC. Adriamycin: amelioration of toxicity by alpha-tocopherol. Cancer Treat Rep 1976;60:961–5.
Myers CE, McGuire WP, Liss RH, Ifrim I, Grotziger K, Young RC. Adriamycin; the role of lipid peroxidation in cardiac toxicity and tumor response. Science 1977;197:165–9.
Sonneveld P. Effect of alpha-tocopoherol on the cardiotoxicity of adriamycin in the rat. Cancer Treat Rep 1978;62:1033–6.
Banks AR, Jones T, Koch TH, Friedman RD, Bachur NR. Prevention of adriamycin toxicity. Cancer Chemother Pharmacol 1983;11:91–3.
Van Vleet JF, Ferrans VJ. Evaluation of vitamin E and selenium protection against chronic adriamycin toxicity in rabbits. Cancer Treat Rep 1980;64:315–7.
Rahman A, Kessler A, More N, et al. Liposomal protection of adriamycin-induced cardiotoxicity in mice. Cancer Res 1980;40:1532–7.
Herman EH, Rahman A, Ferrans VJ, Vick JA, Schein PS. Prevention of chronic doxorubicin cardiotoxicity in beagles by liposomal encapsulation. Cancer Res 1983;43:5427–32.
Forssen EA, Tokes ZA. Use of anionic liposomes for the reduction of chronic doxorubicin-induced cardiotoxicity. Proc Natl Acad Sci USA 1981;78:1873–7.
Forssen EA, Tokes ZA. Improved therapeutic benefits of doxorubicin by entrapment in anionic liposomes. Cancer Res 1983;43:546–50.
Olson F, Mayhew E, Maslow D, Rustum Y, Szoka F. Characterization, toxicity and therapeutic efficacy of adriamycin encapsulated in liposomes. Eur J Cancer Clin Oncol 1982;18:167–76.
Gabizon A, Dagan A, Goren D, Barenholz Y, Fuks Z. Liposomes asin vivo carriers of adriamycin: reduced cardiac uptake and preserved antitumor activity in mice. Cancer Res 1982;42:4734–9.
Mayhew E, Rustum Y, Vail WJ. Inhibition of liver metastases of M 5076 tumor by liposome-entrapped adriamycin. Cancer Drug Deliv 1983;1:43–58.
Van Hoesel QGCM, Steerenberg PA, Crommelin DJA, et al. Reduced cardiotoxicity and nephrotoxicity with preservation of antitumor activity of doxorubicin entrapped in stable liposomes in the Lou/M Wsl rat. Cancer Res 1984;44:3698–705.
Shinozawa S, Araki Y, Oda T. Tissue distribution and antitumor effect of liposome-entrapped doxorubicin (adriamycin) in Ehrlich solid tumor-bearing mouse. Acta Med Okayama 1981;35:395–405.
Bertazolli C, Rovero C, Ballerini L, et al. Experimental systemic toxicology of 4′-epidoxorubicin, a new less cardiotoxic anthracycline antitumor agent. Toxicol Appl Pharmacol 1985;79:412–22.
Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4′-epiadriamycin in mice. Tumori 1985;71:241–9.
Goldin A, Venditti JM, Geran R. Effectiveness of the anthracycline analog 4′-epidoxorubicin in the treatment of experimental tumors: a review. Invest New Drugs 1985;3:3–21.
Sikic BI, Ehsan MN, Harker WG, et al. Dissociation of antitumor potency from anthracycline cardiotoxicity in a doxorubicin analog. Science 1985;228:1544–6.
Arcamone F. Properties of antitumor anthracyclines and new developments in their application: Cain Memorial Award Lecture. Cancer Res 1985;45:5995–9.
Arcamone F, Penco S, Vigevani A, et al. Synthesis and antitumor properties of new glycosides of daunomycinone and adriamycinone. J Med Chem 1975;18: 703–7.
Pettit GR, Einck JJ, Herald CL, et al. The structure of carminomycin. I. J Am Chem Soc 1975;97:7387–8.
Oki T, Kitamura I, Matsuzawa Y, et al. Antitumor anthracycline antibiotics, aclacinomycin A and analogues. II. Structural determination. J Antibiot 1979; 32:801–19.
Oki T. New anthracycline antibiotics. J Antibiot 1977;30(suppl):70–84.
Mathe G, Bayssas M, Gouveia J, et al. Preliminary results of a phase II trial of aclacinomycin in acute leukaemia and lymphosarcoma. Cancer Chemother Pharmacol 1978;1:259–62.
Rothig HJ, Kraemer HP, Sedlacek HH. Aclarubicin: Experimental and clinical experience. Drugs Exp Clin Res 1985;11:123–5.
Ohnuma T, Elias F, Holland JF, Henderson E. Pharmacological and therapeutic efficacy of rubidazone in mice. Comparison with daunomycin and adriamycin. Eur J Cancer 1979;15:363–71.
Maral R. Biological activities of rubidazone. Cancer Chemother Pharmacol 1979;2:31–5.
Baker LH, Kessel DH, Comis RL, Reich SD, DeFuria MD, Crooke ST. American experience with carminomycin. Cancer Treat Rep 1979;63:899–902.
Sartiano GP, Lynch WE, Bullington WD. Mechanism of action of the anthracycline anti-tumor antibiotics, doxorubicin, daunomycin and rubidazone: preferential inhibition of DNA polymerase alpha. J Antibiot 1979;32:1038–45.
DuVernay VH, Pachter JA, Crooke ST. Molecular pharmacological differences between carminomycin and its analog, carminomycin-11-methyl ether, and adriamycin. Cancer Res 1980;40:387–94.
Salmon SE, Liu RM, Casazza AM. Evaluation of new anthracycline analogs with the human tumor stem cell assay. Cancer Chemother Pharmacol 1981;6:103–10.
Kaplan S, Martini A, Varini M, Togni P, Cavalli F. Phase I trial of 4-demethoxydaunorubicin with single i.v. doses. Eur J Cancer Clin Oncol 1982;12:1303–6.
Salmon SE, Young L, Soehnlen B, Liu R. Antitumor activity of esorubicin in human tumor clonogenic assay with comparisons to doxorubicin. J Clin Oncol 1984; 2:282–6.
DiMarco A, Casazza AM, Gambetta R, Supino R, Zunino F. Relationship between activity and amino sugar stereochemistry of daunorubicin and adriamycin derivatives. Cancer Res 1976;36:1962–6.
DiMarco A, Casazza AM, Dasdia T, et al. Changes of activity of daunorubicin, adriamycin and stereoisomers following the introduction or removal of hydroxyl groups in the amino sugar moiety. Chem Biol Interact 1977;19:291–302.
DiMarco A, Arcamone F, Zunino F. Daunomycin (daunorubicin) and adriamycin and structural analogues: biological activity and mechanism of action. In: Corcoran J, Hahn FE, eds. Antibiotics. Vol 3. Berlin-New York: Springer-Verlag, 1974:101–28.
Cox PJ, Farmer PB, Towards selectivity? Approaches to the design of new anti-tumour agents. II. Cancer Treat Rev 1977;4:119–34.
DiMarco A, Zunino F, Casazza AM. Comparison of biochemical and biological methods in the evaluation of new anthracycline drugs. Antibiot Chemother 1978; 23:12–20.
Arcamone F, DiMarco A, Casazza AM. Chemistry and pharmacology of new antitumor anthracyclines. In: Umezawa H, et al., eds. Advances in cancer chemotherapy. Baltimore: University Park Press, 1978:297–312.
Bachur NR. Anthracycline antibiotic pharmacology and metabolism. Cancer Treat Rep 1979;63:817–20.
Casazza AM. Experimental evaluation of anthracycline analogs. Cancer Treat Rep 1979;63:835–44.
Henry DW. Structure-activity relationships among daunorubicin and adriamycin analogs. Cancer Treat Rep 1979;63:845–54.
Brown JR. New natural, semisynthetic and synthetic anthracycline drugs. In: Neidle S, Waring MJ, eds. Molecular aspects of anti-cancer drug action. The Contributors, 1983:57–92.
Arcamone F. Antitumour anthracyclines: recent developments. Med Res Rev 1984;4:153–88.
Formelli F, Casazza AM. Biological and pharmacological properties of new anthracycline derivatives. Drugs Exp Clin Res 1984;10:75–84.
Israel M, Potti GP, Seshadri R. Adriamycin analogues. Rationale, synthesis, and preliminary antitumor evaluation of highly active DNA-nonbindingN-(trifluoroacetyl)adriamycin 14-O-hemiester derivatives. J Med Chem 1985;28:1223–8.
Casazza AM, Pratesi G, Giuliani F, DiMarco A. Antileukemic activity of 4-demethoxydaunorubicin in mice. Tumori 1980;66:548–64.
Streeter DG, Taylor DL, Acton EM, Peters JH. Comparative cytotoxicities of various morpholinyl anthracyclines. Cancer Chemother Pharmacol 1985; 14:160–4.
Neidle S. The molecular basis for the action of some DNA-binding drugs. Progr Med Chem 1979;16:152–221.
Zunino F, DiMarco A, Zaccara A. Molecular structural effects involved in the interaction of anthracyclines with DNA. Chem Biol Interact 1979;24:217–25.
Arlandini E, Vigevani A, Arcamone F. Interaction of new derivatives of daunorubicin and doxorubicin with DNA. Farmaco [Sci] 1980;35:65–78.
Fritzsche H, Triebel H, Chaires JB, Dattagupta N, Crothers DM. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: geometry of intercalation of iremycin and daunomycin. Biochemistry 1982;21:3940–6.
Komiyama T, Oki T, Inui T. Interaction of new anthracycline antibiotics with DNA effects on nucleic acid synthesis and binding to DNA. Biochim Biophys Acta 1983;740:80–7.
Simpkins H, Pearlman LF, Thompson LM. Effects of adriamycin on supercoiled DNA and calf thymus nucleosomes studied with fluorescent probes. Cancer Res 1984;44:613–8.
Forster W, Stutter E. Interaction of anthracycline antibiotics with biopolymers: 9. Comparative study of the interaction kinetics of daunomycin, adriamycin and iremycin with DNA. Int J Biol Macromol 1984;6:114–24.
Berg H. Antitumor anthracyclines — properties and mechanisms. Stud Biophys 1984; 104:13–22.
Neidle S. Interactions of daunomycin and related antibiotics with biological receptors. Top Antibiot Chem 1978;2:242–78.
Donehower RC, Meyers CE, Chabner BA. New developments on the mechanisms of action of antineoplastic drugs. Life Sci 1979;25:1–14.
Gutteridge JMC, Quinlan GJ. Free radical damage to deoxyribose by anthracycline, aureolic acid and aminoquinone antitumour antibiotics. An essential requirement for iron, semiquinones and hydrogen peroxide. Biochem Pharmacol 1985;523:4099–103.
Lown JW, Chen HH, Plambeck JA, Acton EM. Diminished Superoxide anion generation by reduced 5-iminodaunorubicin relative to daunorubicin and relationship to cardiotoxicity of the anthracycline antitumor agents. Biochem Pharmacol 1979;28:2563–8.
Mimnaugh EG, Trush MA, Gram TE. Stimulation by adriamycin of rat heart and liver microsomal NADPH-dependent lipid peroxidation. Biochem Pharmacol 1981;20:2797–804.
Sugioka K, Nakano H, Nakano M, Tero-Kubota S, Ikegami Y: Generation of hydroxyl radicals during the enzymatic reductions of the Fe3+-ADP-phosphateadriamycin and Fe3+-ADP-EDTA systems. Less involvement of hydroxyl radical and a great importance of proposed perferryl ion complexes in lipid peroxidation. Biochim Biophys Acta 1983;753:411–21.
Nohl H, Jordan W. OH Generation by adriamycin semiquinone and H2O2; an explanation for the cardiotoxicity of anthracycline antibiotics. Biochem Biophys Res Commun 1983;114:197–205.
Lown W. Ethidium binding assay for reactive oxygen species generated from reductively activated adriamycin (doxorubicin). Methods Enzymol 1984;105:532–9.
Pollakis G, Goormaghtigh E, Delmelle M, Lion Y, Ruysschaert JM. Adriamycin and derivatives interaction with the mitochondrial membrane: O2 consumption and free radicals formation. Res Commun Chem Pathol Pharmacol 1984;44:445–59.
Dickinson AC, DeJordy JO, Boutin MG, Teres D. Absence of generation of oxygen-containing free radicals with 4′-deoxydoxorubicin, a non-cardiotoxic anthracycline drug. Biochem Biophys Res Commun 1984;125:584–91.
Chinami M, Kato T, Ogura R, Shingu M. Semiquinone formation of adriamycin by oxidation atpara-OH residue. Biochem Int 1984;8:299–304.
Ashnagar A, Bruce JM, Dutton PL, Prince RC. Oneand two-electron reduction of hydroxy-1,4-naphthoquinones and hydroxy-9,10-anthraquinones. The role of internal hydrogen bonding and its bearing on the redox chemistry of the anthracycline antitumor quinones. Biochim Biophys Acta 1984;801:351–9.
Dodd NJF, Mukherjee T. Free radical formation from anthracycline antitumour agents and model systems — I. model naphthoquinones and anthraquinones. Biochem Pharmacol 1984;33:379–85.
Bachur NR. General mechanism for the biological activation of quinone anticancer agents to free radicals. In: Siegenthaler W, Luthy R, eds. Current Chemotherapy. Proceedings of the 10th International Congres on Chemotherapy. Washington DC: American Society for Microbiology, 1978:1124–7.
Sinha BK, Gregory JL. Role of one-electron and two-electron reduction products of adriamycin and daunomycin in deoxyribonucleic acid binding. Biochem Pharmacol 1981:30:2626–9.
Tritton TR, Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science 1982;217:248–50.
Wingard LB, Tritton T, Egler KA. Cell surface effects of adriamycin and carminomycin immobilized on crosslinked polyvinyl alcohol. Cancer Res 1985;45:3529–36.
Goormaghtigh E, Chatelain P, Caspers J, Ruysschaert JM. Evidence of a specific complex between adriamycin and negatively-charged phospholipids. Biochim Biophys Acta 1980;597:1–14.
Arcamone F. Daunomycin and related antibiotics. Top Antibiot Chem 1978;2:102–23.
Remers WA. The chemistry of antitumor antibiotics. Vol. I. New York: John Wiley & Sons, 1979.
Sturgeon RJ, Schulman SG. Electronic absorption spectra and protolytic equilibria of doxorubicin: Direct spectrophotometric determination of microconstants. J Pharm Sci 1977;66:958–61.
Kiraly R, Martin RB. Metal ion binding to daunorubicin and quinizarin. Inorg Chim Acta 1982;67:13–8.
Beraldo H, Garnier-Suillerot A, Tosi L. Copper(II)-adriamycin complexes. A circular dichroism and resonance Raman study. Inorg Chem 1983;22:4117–24.
Righetti PG, Menozzi M, Gianazza E, Valentini L. Protolytic equilibria of doxorubicin as determined by isoelectric focusing and ‘electrophoretic titration curves’. FEBS Lett 1979;101:51–5.
McLennan IJ, Lenkinski RE, Yanuka Y. Nuclear magnetic resonance study of the self-association of adriamycin and daunomycin in aqueous solution. Can J Chem 1985;63:1233–8.
Wilson DW, Grier D, Reimer R, Bauman JD, Preston JF, Gabbau EJ. Structure-activity relationship of daunorubicin and its peptide derivatives. J Med Chem 1976;19:381–4.
Kano K, Konse T, Kubota T. The effects of the pH and the temperature on the oxidation-reduction properties of adriamycin adsorbed on a mercury electrode surface. Bull Chem Soc Jpn 1985;58:424–8.
Brazhnikova MG, Zbarsky VB, Ponomarenko VI, Potapova NP. Physical and chemical characteristics and structure of carminomycin, a new antitumor antibiotic. J Antibiot 1974;27:254–9.
Dalmark M, Storm H. A Fickian diffusion transport process with the features of transport catalysis. J Gen Physiol 1981;78:349–64.
Tanaka M, Yoshida S, Kimura K. Mechanism of inhibition of DNA polymerases by 4′-epiadriamycin and 4′-O-tetrahydropyranyladriamycin. Gann 1983; 74:829–36.
Siegfried JM, Burke TG, Tritton TR. Cellular transport of anthracyclines by passive diffusion. Biochem Pharmacol 1985;34:593–8.
Eksborg S. Extraction of daunorubicin and doxorubicin and their hydroxyl metabolites: self-association in aqueous solution. J Pharm Sci 1978;67:782–5.
Lenkinski RE, Sierke S, Vist MR. Lanthanide complexes of adriamycin. J Less Common Metals 1983;94: 359–65.
Zenebergh A, Baurain R, Trouet A. Cellular pharmacokinetics of aclacinomycin A in cultured L1210 cells. Comparison with daunorubicin and doxorubicin. Cancer Chemother Pharmacol 1982;8:243–9.
Eksborg S. Extraction properties and liquid Chromatographic separation of adriamycin and daunorubicin and their hydroxyl metabolites. Application to bioanalysis. In: Pinedo HM. ed. Clinical Pharmacology of Anti-neoplastic Drugs. Vol I. Amsterdam: Elsevier North Holland/Biomedical Press, 1978:193–207.
Nakazawa H, Riggs CE, Egorin MJ, et al. Continuous extraction of urinary anthracycline antitumor antibiotics with the horizontal flow-through coil planet centrifuge. J Chromatogr 1984;307:323–33.
Yesair DW, Schwartzbach E, Shuck D, Denine EP, Asbell MA. Comparative pharmacokinetics of daunomycin and adriamycin in several animal species. Cancer Res 1972;32:1177–83.
Despois R, Dubost M, Mancy D, et al. Un nouvel antibiotique doué d'activité antitumorale: la rubidomycine (13.057RP). Arzneim Forsch 1967;17:934–9.
Arena E, d'Alessandro N, Dusonchet L, et al. Analysis of the pharmacokinetic characteristics, pharmacological and chemotherapeutic activity of 14-hydroxy-daunomycin (adriamycin), a new drug endowed with an antitumour activity. Arzneim Forsch 1971;21:1258–65.
Bachur NR, Steele M, Meriwether WD, Hildebrand RC. Cellular pharmacodynamics of several anthracycline antibiotics. J Med Chem 1976;19:651–4.
Bachur NR. Biochemical pharmacology of the anthracycline antibiotics. In: Sartorelli AC, ed. Cancer Chemotherapy. Washington DC: American Chemical Society, 1976:58–70.
Supino R, Necco A, Dasdia T, Casazza AM, DiMarco A. Relationship between effects on nucleic acid synthesis in cell cultures and cytotoxicity of 4-demethoxy derivatives of daunorubicin and adriamycin. Cancer Res 1977;37:4523–8.
Yen SF, Wilson WD, Pearce SW, Gabbay EJ. A new series of reductive amination derivatives of daunorubicin: syntheses, partition coefficients, and DNA binding. J Pharm Sci 1984;73:1575–8.
Wilkinson PM, Israel M, Pegg WJ, Frei E. Comparative metabolism and excretion of adriamycin in man, monkey, and rat. Cancer Chemother Pharmacol 1979;2: 121–5.
Terasaki T, Iga T, Sugiyama Y, Hanano M. Pharmacokinetic study on the mechanism of tissue distribution of doxorubicin: interorgan and interspecies variation of tissue-to-plasma partition coefficients in rats, rabbits, and guinea pigs. J Pharm Sci 1984;73:1359–63.
Oosterbaan MJM, Dirks RJM, Vree TB, Van der Kleijn E. Pharmacokinetics of anthracyclines in dogs: evidence for structure-related body distribution and reduction to their hydroxy metabolites. Pharm Res 1984;1:33–8.
Barthelemy-Clavey V, Maurizot JC, Dimicoli JL, Sicard P. Self-association of daunorubicin. FEBS Lett 1974;46:5–10.
Chaires JB, Dattagupta N, Crothers DM. Self-association. Biochemistry 1982;21:3927–32.
Menozzi M, Valentini L, Vannini E, Arcamone F. Self-association of doxorubicin and related compounds in aqueous solution. J Pharm Sci 1984;73:766–70.
Martin SR. Absorption and circular diehroic spectral studies on the self-association of daunorubicin. Biopolymers 1980;29:713–21.
Schutz H, Gollmick FA, Stutter E. Determination of the equilibrium binding parameters of daunomycin to DNA. Stud Biophys 1979;75:147–59.
Stutter E, Gollmick FA, Schutz H. Interaction of anthracycline antibiotics with biopolymers. VI. The binding of adriamycin to DNA under equilibrium conditions. Stud Biophys 1982;88:131–8.
Angerman NS, Victor TA, Bell CL, Danyluk SS. A proton magnetic resonance study of the aggregation of actinomycin D in D2O. Biochemistry 1972;11:2402–11.
Li HJ, Crothers DM. Studies of the optical properties of the proflavine-DNA complex. Biopolymers 1969;8: 217–35.
Dimicoli JL, Helene C. Complex formation between purine and indole derivatives in aqueous solutions. Proton magnetic resonance studies. J Am Chem Soc 1973;95:1036–44.
Muller W, Crothers DM. Interactions of heteroaromatic compounds with nucleic acids. Eur J Biochem 1975;54:267–77.
Tomlinson E, Malspeis L. Concomitant adsorption and stability of some anthracycline antibiotics. J Pharm Sci 1982;71:1121–5.
Speth PAJ, Linssen PCM, Boezeman JBM, Wessels HMC, Haanen C. Quantitation of anthracyclines in human hematopoietic cell subpopulations by flow cytometry correlated with high pressure liquid chromatography. Cytometry 1985;6:143–50.
Bots AMB, Van Oort WJ, Noordhoek J, Van Dijk A, Klein SW, Van Hoesel QGCM. Analysis of adriamycin and adriamycinol in micro volumes of rat plasma. J Chromatogr 1983;272:421–7.
Eksborg S. Reversed-phase liquid chromatography of adriamycin and daunorubicin and their hydroxyl metabolites. J Chromatogr 1978;149:225–32.
Eksborg S, Ehrsson H, Andersson B, Beran M. Liquid Chromatographic determination of daunorubicin and daunorubicinol in plasma from leukemic patients. J Chromatogr 1978;153:211–8.
Moro E, Bellotti V, Jannuzzo MG, Stegnjaich S, Valzelli G. High-performance liquid Chromatographic method for pharmacokinetic studies of the new anthracycline 4-demethoxydaunorubicin and its 13-dihydro derivative. J Chromatogr 1983;274:281–7.
Stanton GF, Raymond V, Wittes RE, et al. Phase 1 and clinical pharmacological evaluation of 4′-deoxydoxorubicin in patients with advanced cancer. Cancer Res 1985;45:1862–8.
Chan KK, Harris PA. A fluorometric determination of adriamycin and its metabolites in biological tissues. Res Commun Chem Pathol Pharmacol 1973;6:447–63.
Andrews PA, Brenner DE, Chou FT, Kubo H, Bachur NR. Facile and definitive determination of human adriamycin and daunorubicin metabolites by highpressure liquid chromatography. Drug Metab Dispos 1980;8:152–6.
Benvenuto JA, Anderson RW, Kerkof K, Smith RG, Loo TL. Stability and compatibility of antitumor agents in glass and plastic containers. Am J. Hosp Pharm 1981;38:1914–8.
Storm G, Van Bloois L, Brouwer M, Crommelin DJA. The interaction of cytostatic drugs with adsorbents in aqueous media. The potential implications for liposome preparation. Biochim Biophys Acta 1985;818:343–51.
Kano K, Konse T, Nishimura N, Kubota T. Electrochemical properties of adriamycin adsorbed on a mercury electrode surface. Bull Chem Soc Jpn 1984; 57:2383–90.
Kano K, Konse T, Kubota T. The curve fitting analysis of d.c. and a.c. voltammograms of a two-step surfaceredox reaction. The application to the surface-redox system of adriamycin adsorbed on a pyrolytic graphite electrode. Bull Chem Soc Jpn 1985;58:1879–85.
Mebsout F, Vire JC, Patriarche GJ. Compartement polarographique d'un nouvel agent cytostatique: la carminomycine. Anal Lett 1984;17:805–16.
Baldwin RP, Packett D, Woodcock TM. Electrochemical behavior of adriamycin at carbon paste electrodes. Anal Chem 1981;53:540–2.
Chaney EN, Baldwin RK. Electrochemical determination of adriamycin compounds in urine by preconcentration at carbon paste electrodes. Anal Chem 1982;54:2556–60.
Greenaway FT, Dabrowiak JC. The binding of copper ions to daunomycin and adriamycin. J Inorg Biochem 1982;16:91–107.
Mariam YH, Wells W. Yb(III)-daunomycin interactions. Determination of nature and number of species by matrix rank analysis. J Sol Chem 1984;13:269–80.
Beraldo H, Garnier-Suillerot A, Tosi L, Lavelle F. Iron(III)-adriamycia and iron(III)-daunorubicin complexes: physicochemical characteristics, interaction with DNA, and antitumor activity. Biochemistry 1985;24:284–9.
Muindi JRF, Sinha BK, Gianni L, Meyers CE. Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett 1984; 172:226–30.
McLennan IJ, Lenkinski RE. The binding of Yb(III) to adriamycin. A proton NMR relaxation study. J Am Chem Soc 1984;106:6905–9.
Fiallo MML, Garnier-Suillerot A. Physicochemical studies of the iron(III)-carminomycin complex and evidence of the lack of stimulated Superoxide production by NADH dehydrogenase. Biochim Biophys Acta 1985;840:91–8.
Kozlowski H, Drabent K, Szyszuk H, Gosalvez M. Mossbauer, magnetic and-thermogravimetric studies of adriamycin ferric complexes. Inorg Chim Acta 1982;66:189–92.
May PM, Williams GK, Williams DR. Speciation studies of adriamycin, quelamycin and their metal complexes. Inorg Chim Acta 1980;46:221–8.
Budevsky O. Foundations of chemical analysis. Chichester: Ellis Horwood Ltd., 1979:353–8.
Phillips DR, Carlyle GA. The effect of physiological levels of divalent metal ions on the interaction of daunomycin with DNA: evidence of a ternary daunomycin-Cu2+-DNA complex. Biochem Pharmacol 1981;30:2021–4.
Spinelli M, Dabrowiak JC. Interaction of copper(II) ions with the daunomycin-calf thymus deoxyribonucleic acid complex. Biochemistry 1982;21:5862–70.
Fishman MM, Schwartz I. Effect of divalent cations on the daunomycin-deoxyribonucleic acid complex. Biochem Pharmacol 1974;23:2147–54.
Elliot H, Gianni L, Myers C. Oxidative destruction of DNA by the adriamycin-iron complex. Biochemistry 1984;23:928–36.
Nakano H, Ogita K, Gutteridge JMC, Nakano M. Inhibition by the protein ceruloplasmin of lipid peroxidation stimulated by an Fe3+-ADP-adriamycin complex. FEBS Lett 1984;166:232–6.
Mikelens P, Levinson W. Metal ion participation in binding of daunomycinone, daunomycin, and adriamycin to nucleic acids. Bioinorg Chem 1978;9:441–52.
Gutteridge JMC. Lipid peroxidation and possible hydroxyl radical formation stimulated by the self-reduction of a doxorubicin-iron(III) complex. Biochim Pharmacol 1984;33:1725–8.
Zweier JL. Reduction of O2 by iron-adriamycin. J Biol Chem 1984;259:221–8.
Myers CE, Gianni L, Simone CB, Klecker R, Greene R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry 1982;21:1707–13.
Sugioka K, Nakano M. Mechanism of phospholipid peroxidation induced by ferric ion-ADP-adriamycinco-ordination complex. Biochem Biophys Acta 1982;713:333–43.
Demant EJ. Binding of adriamycin-Fe3+ complex to membrane phospholipids. Eur J Biochem 1984;142: 571–5.
Gosalvez M. Metal chelate derivatives of anthracycline antibiotics. US Patent 1977;21.01.77.
Gosalvez M, Blanco FF, Vivero C, Valles F. Quelamycin, a new derivative of adriamycin with several possible therapeutic advantages. Eur J Cancer 1978;14:1185–90.
Porumb H. The solution spectroscopy of drugs and the drug-nucleic acid interactions. Prog Biophys Mol Biol 1978;34:175–95.
DiMarco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123, 127): A new antibiotic with antitumor activity. Cancer Chemother Rep 1969;53:33–7.
Oki T, Matsuzawa Y, Yoshimoto A, et al. New antitumor antibiotics aclacinomycins A and B. J Antibiot 1975;28:830–4.
Ando T, Hirayama K, Takahashi R, et al. Cosmomycin D, a new anthracycline antibiotic. Agric Biol Chem 1985;49:259–62.
Ando T, Hirayama K, Takahashi R, et al. The structure of anthracycline antibiotics cosmomycins A and B. Agric Biol Chem 1985;49:1207–9.
Mailer K, Petering DH. Inhibition of oxidative phosphorylation in tumor cells and mitochondria by daunomycin and adriamycin. Biochim Pharmacol 1976;25: 2085–9.
Plummier-van den Bussche D, Dryon L. Colorimetrische gehaltebepaling van daunomycine HCl. Farm Tijdschr Belg 1976;53:133–40.
Calendi E, DiMarco A, Reggiani M, Scarpinato B, Valentini L. On physico-chemical interactions between daunomycin and nucleic acids. Biochim Biophys Acta 1965;103:25–49.
Youngman RJ, Elstner EF. On the interaction of adriamycin with DNA: investigation of spectral changes. Arch Biochem Biophys 1984;231:424–9.
DiMarco A, Zunino F, Silvestrini R, Gambarucci C, Gambetta RA. Interaction of some daunomycin derivatives with deoxyribonucleic acid and their biological activity. Biochem Pharmacol 1971;20:1323–8.
Land EJ, Mukherjee T, Swallow AJ, Bruce JM. Possible intermediates in the action of adriamycin — A pulse radiolysis study. Br J Cancer 1985;51:515–23.
Bachur NR, Moore AL, Bernstein JG, Liu A. Tissue distribution and disposition of daunomycin in mice: Fluorometric and isotopic methods. Cancer Chemother Rep 1970;54:89–94.
Ganapathi R, Gulick P, Miller R, et al. Analysis of heterogeneity in daunorubicin uptake by human leukemia cells using laser flow cytometry. Invest New Drugs 1985;3:273–7.
Nooter K, Sonneveld P, Martens A. Differences in the pharmacokinetics of daunomycin in normal and leukemic rats. Cancer Res 1985;45:4020–5.
Krishan A, Ganapathi R. Laser flow cytometric studies on the intracellular fluorescence of anthracyclines. Cancer Res 1980;40:3895–900.
Nicolo G, Esposito M, Beicastro A, Rovida S, Margallo E, Santi L. Cytophotometric analysis of the uptake of adriamycin inin vivo normal mouse liver and kidney cells. Anal Quant Cytol 1982;4:295–301.
Chambers SH, Bleehen NM, Watson JV. Effect of cell density on the intracellular adriamycin concentration and cytotoxicity in exponential and plateau phase EMT6 cells. Br J Cancer 1984;49:301–6.
Paschoud N, Bignell C, Reinhold HS. A double fluorescence method for determining doxorubicin distribution and vascular supply in the mouse kidney. J Histochem Cytochem 1985;33:73–6.
Tromberg BJ, Eastham JF, Sepaniak MJ. Optical fiber fluoroprobes for biological measurements. Appl Spectroscopy 1984;38:38–42.
Dusonchet L, Gebbia N, Gerbasi F. Spectrophotofluorometric characterization of adriamycin, a new anti-tumour drug. Pharmacol Res Commun 1971;3:55–65.
Pasqualino A, Picone MA, Traine A. Spectrofluorophotometric determination of daunomycin in the tissues. Arzneim Forsch 1969;19:774–6.
Tavaloni N, Guarino AM. Biliary and urinary excretion of adriamycin in anesthetized rats. Pharmacology 1980;10:256–67.
Tavoloni N, Guarino AM. Bile secretory function: a determinant of adriamycin disposition. Arch Int Pharmacodyn 1980;245:180–97.
Sonneveld P, Van Bekkum DW. Different distribution of adriamycin in normal and leukaemic rats. Br J Cancer 1981;43:464–70.
Chang BK. Tissue distribution of adriamycin administered intraperitoneally vs intravenously, with special emphasis on the pancreas. Bull Cancer 1982;69:172–5.
Bachur NR, Riggs CE, Green MR, Langone JJ, Van Vunakis H, Levine L. Plasma adriamycin and daunorubicin levels by fluorescence and radioimmunoassay. Clin Pharmacol Ther 1977;21:70–7.
Formelli F, Pollini C, Casazza AM, DiMarco A, Mariani A. Fluorescence assays and pharmacokinetic studies of 4′-deoxydoxorubicin and doxorubicin in organs of mice bearing solid tumors. Cancer Chemother Pharmacol 1981;5:139–44.
Nguyen-Ngoc T, Vrignaud P, Robert J. Cellular pharmacokinetics of doxorubicin in cultured mouse sarcoma cells originating from autochthonous tumors. Oncology 1984;41:55–60.
DuVernay VH, Pachter JA, Crooke ST. DNA binding studies on several new anthracycline antitumor antibiotics. II. The importance of the carbomethoxy-group at position-10 of the class II anthracycline molecule. Mol Pharmacol 1979;16:623–32.
Valentini L, Nicolella V, Vannini E, Menozzi M, Penco S, Arcamone F. Association of anthracycline derivatives with DNA: A fluororescence study. Farmaco [Sci] 1985;40:377–90.
Takanashi S, Bachur NR. Adriamycin metabolism in man. Evidence from urinary metabolites. Drug Metab Dispos 1976;4:79–87.
Takanashi S, Bachur NR. Daunorubicin metabolites in human urine. J Pharmacol Exp Ther 1975;195:41–9.
Bullock FJ, Bruni RJ, Asbell MA. Identification of new metabolites of daunomycin and adriamycin. J Pharmacol Exp Ther 1972;182:70–6.
Bachur NR. Daunorubicinol, a major metabolite of daunorubicin: isolation from human urine and enzymatic reactions. J Pharmacol Exp Ther 1971;177:573–8
Marshall VP, Reisender EA, Reineke LH, Johnson JH, Wiley PF. Reductive microbial conversion of anthracycline antibiotics. Biochemistry 1976;15:4139–45.
Karnetova J, Mateju J, Sedmera P, Vokoun J, Vanek Z. Microbial transformation of daunomycinone byStreptomyces aureofaciens B-96. J Antibiot 1976;29: 1199–202.
Vigevani A, Williamson MJ. Doxorubicin. Anal Prof Drug Subst 1980;9:245–74.
Manfait M, Theophanides T. Fourier transform infrared spectra of cells treated with the drug adriamycin. Biochem Biophys Res Commun 1983;116:321–6.
Arnone A, Fronza G, Mondelli R, Vigevani A.13C-NMR analysis of the antitumor antibiotics daunorubicin and adriamycin. Tetrahedron Lett 1976;37:3349–50.
Arcamone F, Franceschi G, Orezzi P, Penco S, Mondelli R. The structure of daunomycin. Tetrahedron Lett 1968;30:3349–52.
Wiley PF, Kelly RB, Caron EL, et al. Structure of nogalamycin. J Am Chem Soc 1977;99:542–9.
Penco S, Cassinelli G, Vigevani A, Zini P, Rivola G, Arcamone F. Daunorubicin aldo-keto reductases: enantioface differential reduction of the side-chain carbonyl group of antitumor anthracyclines. A correction of the stereochemistry at C(13) of 4-demethoxy-13-dihydrodaunorubicin. Gazz Chim Ital. 1985; 115:195–7.
Paulick RC, Casey ML, Whitlock FJ. A13C nuclear magnetic resonance study of the biosynthesis of daunomycin from13CH3 13CO2Na. J Am Chem Soc 1976; 98:3370–1.
Arcamone F, Cassinelli G, Franceschi G, et al. Part 1. Chemistry of adriamycin. Structure and physicochemical properties of adriamycin (doxorubicin). In: Carter SK, DiMarco A, Ghione M, Krakoff IN, Mathe G, eds. International Symposium on Adriamycin. Berlin: Springer-Verlag, 1972:9–22.
Brockmann H, Budzikiewicz H, Djerassi C, Brockmann H, Niemeyer J. Das massenspektroskopische Fragmentierungsverhalten der Anthracyclinone. Chem Ber 1965;98:1260–9.
Nakazawa H, Andrews PA, Bachur NR, Ito Y. Isolation of daunorubicin derivatives by counter-current chromatography with the horizontal flow-through coil planet centrifuge. J Chromatogr 1981;205:482–5.
Watson E, Chan KK. GLC-mass spectrometry of several important anticancer drugs. I: pertrimethylsilylation andO-methoxime formation. J Pharm Sci 1978;67:1243–6.
Chan KK, Watson E. GLC-mass spectrometry of several important anticancer drugs. II: doxorubicin and daunorubicin aglycone analogs. J Pharm Sci 1978; 67:1748–52.
Roller PP, Sutphin M, Aszalos AA. Mass spectrometry ofN-acylated daunorubicin derivatives. Biomed Mass Spectrom 1976;3:11–71.
Vigevani A, Gioia B, Cassinelli G. Mass spectrometry of someN-acyldaunosamine derivatives. Carbohydr Res 1974;32:321–30.
Andrews PA, Callery PS, Chou FE, May ME, Bachur NR. Qualitative analysis of trimethylsilylated daunosamine andN-alkylated analogs by gas chromatography-mass spectrometry. Anal Biochem 1982; 126:258–67.
Beijnen JH, Wiese G, Underberg WJM. Aspects of the chemical stability of doxorubicin and seven other anthracyclines in acidic solution. Pharm Weekbl [Sci] 1985;7:109–16.
Smith RG. Characterization of anthracycline antibiotics by desorption chemical ionization mass spectrometry. Anal Chem 1982;54:2006–8.
Gioia B, Arlandini E, Vigevani A. Field desorption mass spectrometry of anthracyclines. Comparison with other soft ionization techniques. Biomed Mass Spectrom 1984;11:35–40.
Cassinelli G, Configliacchi E, Penco S, et al. Separation, characterization, and analysis of epirubicin (4′-epidoxorubicin) and its metabolites from human urine. Drug Metab Dispos 1984;12:506–10.
Roboz J. Applications of combined gas chromatography-mass spectrometry in clinical chemistry. In: Simmons IL, Ewing GW, eds. Progress in Analytical Chemistry. Applications of the newer techniques of analysis. Vol. 6. New York-London: Plenum Press, 1973:291–5.
Shinozawa S, Mimaki Y, Araki Y, Oda T. Determination of the concentration of adriamycin and its metabolites in the serum and tissues of Ehrlich carcinoma-bearing mice by high-performance liquid chromatography. J Chromatogr 1980;196:463–9.
Acton EM, Tong GL, Mosher CW, Wolgemuth RL. Intensely potent morpholinyl anthracyclines. J Med Chem 1984;27:638–45.
Yoshimoto A, Matsuzawa Y, Ishikura T, Sawa T, Takeuchi T, Umzawa H. New anthracycline derivatives from betaclamycin A. J Antibiot 1984;37:920–2.
Mateju J, Beran M, Jizba J, Podojil M. TLC and HPLC of mixture of anthracyclinones. J Liq Chromatogr 1981;4:977–83.
Beijnen JH, Van der Nat JM, Labadie RP, Underberg WJM. Decomposition of mitomycin and anthracycline cytostatics in cell culture media. Anticancer Res 1986;6:39–44.
Rahman A, Goodman A, Foo W, Harvey J, Smith FP, Schein PS. Clinical pharmacology of daunorubicin in phase I patients with solid tumors: development of an analytical methodology for daunorubicin and its metabolites. Semin Oncol 1984;11:36–44.
Israel M, Pegg WJ, Wilkinson PM, Garnick MB. Liquid Chromatographic analysis of adriamycin and metabolites in biological fluids. J Liq Chromatogr 1978;1:795–809.
Watson E, Chan KK. Rapid analytic method for adriamycin and metabolites in human plasma by a thin-film fluorescence scanner. Cancer Treat Rep 1976;60:1611–8.
Harris PA. Phenobarbital andC. parvum effects on adriamycin elimination. Proc Am Assoc Cancer Res 1976;16:131.
Barbieri B, Abbruzzi R, Benigni A, et al. Quantitative thin-layer Chromatographic measurements ofN-trifluoroacetyladriamycin-14-valerate (AD32) andN-trifluoroacetyladriamycin (AD41) in blood and tissues. J Chromatogr 1979;163:195–200.
Chan KK, Wong CD. Quantitative thin-layer chromatography: thin-film fluorescence scanning analysis of adriamycin and metabolites in tissue. J Chromatogr 1979;172:343–9.
Lee YN, Chan KK, Harris PA, Cohen SL. Distribution of adriamycin in cancer patients. Tissue uptakes, plasma concentration after IV and hepatic IA administration. Cancer 1980;45:2231–9.
Colombo T, Broggini M, Garattini S, Donelli MG. Differential adriamycin distribution to blood components. Eur J Drug Metab Pharmacokinet 1981;6: 115–22.
Terasaki T, Iga T, Sugiyama Y, Hanano M. Experimental evidence of characteristic tissue distribution of adriamycin. Tissue DNA concentration as a determinant. J Pharm Pharmacol 1982;34:597–600.
Asbell MA, Schwartzbach E, Bullock FJ, Yesair DW. Daunomycin and adriamycin metabolism via reductive cleavage. J Pharmacol Exp Ther 1972;182:63–9.
Cradock JC, Egorin MJ, Bachur NR. Daunorubicin bilary excretion and metabolism in the rat. Arch Intern Pharmacodyn 1973;202:48–61.
Benjamin RS, Riggs CE, Bachur NR. Pharmacokinetics and metabolism of adriamycin in man. Clin Pharmacol Ther 1973;14:592–600.
Bachur NR, Hildebrand RC, Jaenke RS. Adriamycin and daunorubicin disposition in the rabbit. J Pharmacol Exp Ther 1974;191:331–40.
Reich SD, Bachur NR. Alterations in adriamycin efficacy by phenobarbital. Cancer Res 1976;36:3803–6.
Reich SD, Steinberg F, Bachur NR, Riggs CE, Goebel R, Berman M. Mathematical model for adriamycin (doxorubicin) pharmacokinetics. Cancer Chemother Pharmacol 1979;3:125–31.
Tavoloni N, Guarino AM. Disposition and metabolism of adriamycin in the rat. Pharmacology 1980;21: 244–55.
Ehninger G, Stocker HJ, Proksch B, Wilms K. Die Pharmakokinetik von Adriamycin und Adriamycin-Metaboliten. Klin Wochenschr 1980;58:927–34.
Egorin MJ, Clawson RE, Ross LA, et al. Murine metabolism and disposition of iron:adriamycin complexes. Cancer Res 1983;43:3253–62.
Galloway SJ, Brenner DE, Cooper J, Noone R, Forbes J, Hande KR. A comparison of thin layer chromatography (TLC) and high pressure liquid chromatography (HPLC) for the assay of adriamycin (A1) and its metabolites (metabs). Proc Am Assoc Cancer Res 1983;24:257.
Brenner DE, Galloway S, Cooper J, Noone R, Hande KR. Improved high-performance liquid chromatography assay of doxorubicin: detection of circulating aglycones in human plasma and comparison with thin-layer chromatography. Cancer Chemother Pharmacol 1985;14:139–45.
Benjamin RS, Riggs CE, Bachur NR. Plasma pharmacokinetics of adriamycin and its metabolites in humans with normal hepatic and renal function. Cancer Res 1977;37:1416–20.
Barth HG, Connor AZ. Determination of doxorubicin hydrochloride in pharmaceutical preparations using high-pressure liquid chromatography. J Chromatogr 1977;131:375–81.
Israel M, Wilkinson PM, Pegg WJ, Frei E. Hepatobilary metabolism and excretion of adriamycin andN-trifluoracetyladriamycin-14-valerate in the rat. Cancer Res 1978;38:365–70.
Andersson B, Beran M, Eberhardsson B, Eksborg S, Slanina P. Uptake and distribution of daunorubicin-DNA complex in mice as studied by whole-body autoradiography and liquid chromatography. Cancer Chemother Pharmacol 1979;2:159–67.
Arcamone F, Lazzati M, Vicario GP, Zini G. Disposition of14C-labeled 4′-epidoxorubicin and doxorubicin in the rat. A comparative study. Cancer Chemother Pharmacol 1984;12:157–66.
Issaq HJ, Risser NH, Aszalos A. Thin-layer Chromatographic separation and quantitation of the antitumor agent daunorubicin in fermentation media. J Liq Chromatogr 1979;2:533–8.
Dornberger K, Stengel C, Miosga N. Thin-layer Chromatographic analysis of adriamycinone in fermentation broths. J Chromatogr 1985;328:432–5.
Pandey RC, Toussaint MW. High-performance liquid chromatography and thin-layer chromatography of anthracycline antibiotics. Separation and identification of components of the daunorubicin complex from fermentation broth. J Chromatogr 1980;198:407–20.
Israel M, Pegg WJ, Wilkinson PM, Garnick MB. HPLC applications in the analysis of adriamycin and analogs in biological fluids. In: Hawk GL, ed. Biological/Biomedical Applications of Liquid Chromatography. New York: Marcel Dekker Inc., 1979:413–28. (Chromatographic Science Series. Vol. 10.)
Robert J, Iliadis A, Hoerni B, Cano JP, Durand M, Lagarde C. Pharmacokinetics of adriamycin in patients with breast cancer: Correlation between pharmacokinetic parameters and clinical short-term response. Eur J Cancer Clin Oncol 1982;18:739–45.
Cummings J, Stuart JFB, Caiman KC. Determination of adriamycin, adriamycinol and their 7-deoxy aglycones in human serum by high-performance liquid chromatography. J Chromatogr 1984;311:125–33.
Kovach JS, Ames MM, Sternad ML, O'Connel MJ, Phase I trial and assay of rubidazone (NSC 164011) in patients with advanced solid tumors. Cancer Res 1979;39:823–8.
Aszalos A. Analysis of antitumor antibiotics by high pressure liquid chromatography (HPLC). J Liq Chromatogr 1984;7(S-1):69–125.
Sadee W, El Sayed YM. Antineoplastic drugs. In: Kabra PM, Marton LJ, eds. Liquid Chromatography in Clinical Analysis. Clifton (NJ): The Humana Press, 1980:211–21.
Eksborg S, Ehrsson H. Drug level monitoring: cytostatics. J Chromatogr 1985;340:31–72.
Marshall VP, Reisender EA, Wiley PF. Bacterial metabolism of daunomycin. J Antibiot 1976;24:966–8.
Roboz J, Jacobs AJ, Holland JF, Deppe G, Cohen CJ. Intraperitoneal infusion of doxorubicin in the treatment of gynecologic carcinomas. Med Pediatr Oncol 1981;9:245–50.
Averbuch SD, Finkelstein TT, Fandrich SE, Reich SD. Anthracycline assay by high-pressure liquid chromatography. J Pharm Sci 1981;70:265–70.
Baurain R, Zenebergh A, Trouet A. Cellular uptake and metabolism of daunorubicin as determined by high-performance liquid chromatography. Application to L1210 cells. J Chromatogr 1978;157:331–6.
Baurain R, Masquelier M, Peterson C, Deprez-DeCampeneere D, Trouet A. Quantitative determination of intracellular concentrations of daunorubicin, adriamycin and their main metabolites by high-pressure liquid chromatography. In: Siegenthaler W, Luthy R, eds. Current Chemotherapy. Proceedings of the 10th International Congress on Chemotherapy. Washington DC: American Society for Microbiology, 1978:1130–2.
Ogasawara T, Goto S, Mori S, Oki T. High performance liquid Chromatographic determination of aclacinomycin A and its related compounds. I. Normal phase HPLC for monitoring fermentation and purification processes. J Antibiot 1981;34:47–51.
Peters JH, Murray JF. Determination of adriamycin and aclacinomycin A in plasma by high pressure liquid chromatography and spectrofluorometry. J Liq Chromatogr 1979;2:45–52.
Baurain R, Deprez-DeCampeneere D, Trouet A. Determination of daunorubicin, doxorubicin and their fluorescent metabolites by high-pressure liquid chromatography: plasma levels in DBA2 mice. Cancer Chemother Pharmacol 1979;2:11–4.
Baurain R, Deprez-DeCampeneere D, Trouet A. Rapid determination of doxorubicin and its fluorescent metabolites by high pressure liquid chromatography. Anal Biochem 1979;94:112–6.
Baurain R, Deprez-DeCampeneere D, Trouet A. Distribution and metabolism of rubidazone and daunorubicin in mice. Cancer Chemother Pharmacol 1979;2:37–41.
Peters JH, Gordon GR, Kashiwase D, Acton EM. Tissue distribution of doxorubicin and doxorubicinol in rats receiving multiple doses of doxorubicin. Cancer Chemother Pharmacol 1981;7:65–9.
Londos-Gagliardi D, Baurain R, Robert J, Aubel-Sadron G. Metabolism of daunorubicin in sensitive and resistant Ehrlich ascites tumor cells. Determination by high pressure liquid chromatography. Cancer Chemother Pharmacol 1982;9:45–8.
Ichiba H, Morishita M, Yajima T, et al. Determination of adriamycin and its fluorescent metabolites in biological fluids of inpatients with lung cancer by high performance liquid chromatography. Chem Pharm Bull 1985;33:3868–74.
Hulhoven R, Desager JP. Quantitative determination of low levels of daunomycin and daunomycinol in plasma by high pressure liquid chromatography. J Chromatogr 1976;125:369–74.
Hulhoven R, Desager JP. HPLC determination of daunorubicin and daunorubicinol in human plasma. Biomedicine 1977;27:102–4.
Hulhoven R, Desager JP, Harvengt C. HPLC determination of rubidazone and metabolites. Cancer Chemother Pharmacol 1979;3:133.
Nettleton DE, Balitz DM, Doyle TW, et al. Antitumor agents from bohemic acid complex, III. The isolation of marcellomycin, mussettamycin, rudolphomycin, mimimycin, collinemycin, alcindoromycin, and bohemamine. J Nat Prod 1980;43:242–58.
Shinozawa S, Mimaki Y, Araki Y, Oda T. Determination of the concentration of adriamycin and its metabolites in the serum and tissues of Ehrlich carcinoma bearing mice by high-performance liquid chromatography. J Chromatogr 1980;196:463–9.
Mimaki Y, Tomano H, Shinozawa S, Fukuda T, Araki Y. Behaviour of adriamycin and its metabolites in Ehrlich carcinoma-bearing mice by use of high-performance liquid chromatography with a fluorescence detector. Gan To Kagaku Ryoho 1981;8:86–92.
Shinozawa S, Oda T. Determination of adriamycin (doxorubicin) and related fluorescent compounds in rat lymph and gall by high-performance liquid chromatography. J Chromatogr 1981;212:323–30.
Shinozawa S, Fukuda T, Araki Y, Oda T. Pharmacokinetic analysis of adriamycin (doxorubicin) and related fluorescent compounds in Ehrlich tumor-bearing mouse. Acta Med Ogayama 1982;36:125–32.
Schwartz HS, Parker NB. Initial biotransformations of daunorubicin to aglycones by rat liver microsomes. Cancer Res 1980;41:2343–8.
Schwartz HS, Paul B. Biotransformations of daunorubicin aglycones by rat liver microsomes. Cancer Res 1984;44:2480–4.
Haneke AC, Crawford J, Aszalos A. Quantitation of daunorubicin, and their aglycones by ion-pair reversed phase chromatography. J Pharm Sci 1981;70:112–5.
Wassermann K, Bundgaard H. Kinetics of the acid-catalyzed hydrolysis of doxorubicin. Int J Pharm 1983;14:73–8.
Sepaniak MJ, Yeung ES. Determination of adriamycin and daunorubicin in urine by high-performance liquid chromatography with laser fluorimetric detection. J Chromatogr 1980;190:377–83.
Weenen H, Lankelma J, Penders PGM, et al. Pharmacokinetics of 4′-epidoxorubicin in man. Invest New Drugs 1983;1:59–64.
Weenen H, Van Maanen JMS, De Planque MM, McVie JG, Pinedo HM. Metabolism of 4′-modified analogs of doxorubicin. Unique glucuronidation pathway for 4′-epidoxorubicin. Eur J Clin Oncol 1984;20:919–26.
Brown JE, Wilkinson PA, Brown JR. Rapid highperformance liquid Chromatographic assay for the anthracyclines daunorubicin and 7-con-O-methylnogarol in plasma. J Chromatogr 1981;226:521–5.
Oosterbaan MJM, Dirks RJM, Vree TB, Van der Kleijn E. Rapid quantitative determination of seven anthracyclines and their hydroxy metabolites in body fluids. J Chromatogr 1984;306:323–32.
Eksborg S, Ehrsson H, Andersson I. Reversed-phase liquid Chromatographic determination of plasma levels of adriamycin and adriamycinol. J Chromatogr 1979;164:479–86.
Eksborg S, Ehrsson H. Liquid chromatography in anticancer drug research with special reference to anthraquinone glycosides. J Pharm Biomed Anal 1984;2:297–303.
Pierce RN, Jatlow PI. Measurement of adriamycin (doxorubicin) and its metabolites in human plasma using reversed-phase high-performance liquid chromatography and fluorescence detection. J Chromatogr 1979;164:471–8.
Poochikian GK, Cradock JC, Flora KP. Stability of anthracycline agents in four infusion fluids. Am J Hosp Pharm 1981;38:483–6.
Moro E, Jannuzzo MG, Ranghieri M, Stegnjaich S, Valzelli G. Determination of 4′-epidoxorubicin and its 13-dihydro derivative in human plasma by high-performance liquid chromatography. J Chromatogr 1982;230:207–11.
Lankelma J, Penders PGM, McVie JG, et al. Plasma concentrations of carminomycin and carminomycinol in man, measured by high pressure liquid chromatography. Eur J Cancer Clin Oncol 1982;18:363–7.
Anonymous. Code of Federal Regulations 23. Food and Drug 450.22, 1982.
DeGregorio MW, Ming Lui G, Macher BA, Wilbur JR. Uptake, metabolism, and cytotoxicity of doxorubicin in human Ewings' sarcoma and rhabdomyosarcoma cells. Cancer Chemother Pharmacol 1984;12:59–63.
Kaplan S, Sessa C, Willems Y, Pacciarini MA, Tamassia V, Cavalli F. Phase I trial of 4-demethoxydaunorubicin (idarubicin) with single oral doses. Invest New Durgs 1984;2:281–6.
Alemanni A, Riedmann M. HPLC routine analysis of biosynthetic active compounds in fermentation media. Chromatographia 1979;12:396–8.
Robert J, Extraction of anthracyclines from biological fluids for HPLC evaluation. J Liq Chromatogr 1980; 3:1561–72.
Robert J, Iliadis A, Hoerni B, Cano JP, Durand M, Lagarde C. Pharmacokinetics of adriamycin in patients with breast cancer: Correlation between pharmacokinetic parameters and clinical short-term response. Eur J Clin Oncol 1982; 18:739–45.
Karlsen J, Thonessen HH, Olsen IR, Sollien AH, Skobba TJ. Stability of cytotoxic intravenous solutions subjected to freeze-thaw treatment. Nord Pharm Acta 1983;45:61–7.
Gil P, Favre R, Durand A, Iliadis S, Carcassonne Y. Time dependency of adriamycin and adriamycinol kinetics. Cancer Chemother Pharmacol 1983;19:120–4.
Bolanowska W, Gessner T, Preisler H. A simplified method for determination of daunorubicin, adriamycin, and their chief fluorescent metabolites in human-plasma by high-pressure liquid chromatography. Cancer Chemother Pharmacol 1983;10:187–91.
Matsushita Y, Iguchi H, Kiyosaka T, et al. A high performance liquid Chromatographic method of analysis of 4′-O-tetrahydropyranyladriamycin and their metabolites in biological samples. J Antibiot 1983;36: 880–6.
Ogasawara T, Masuda Y, Goto S, Mori S, Oki T. High performance liquid Chromatographic determination of aclacinomycin A and its metabolites in biological fluids using fluorescence detection. J Antibiot 1981;34:52–7.
Rahmani R, Gil P, Martin M, Durand A, Babet J, Cano JP. Quantitation of adriamycin in plasma and urine: comparative study of radioimmunoassay and high-performance liquid chromatography. J Pharm Biomed Anal 1983;1:301–9.
Peng YM, Alberts DS, Salmon SE, Davis TP. A method for the simultaneous measurement of the new anthracycline derivative 4′-deoxydoxorubicin and its metabolites by reversed phase liquid chromatography. Invest New Drugs 1984;2:277–80.
Alemanni A, Breme U, Vigevani A. Determination of anthracyclines in fermentation broths by HPLC. Process Biochem 1982:9–12.
Pizzorno G, Trave F, Mazzoni A, Russello O, Nicolin A. Contemporary detection of 4-demethoxydaunorubicin and its metabolites 13-dihydro-4-demethoxydaunorubicin and 4-demethoxydaunorubicinone by reversed phase high-performance liquid chromatography. J Liq Chromatogr 1985;8:2557–66.
Quattrone AJ, Ranney DF. Simplified toxicologic monitoring of adriamycin, its major metabolites and nogalamycin by reversed-phase high pressure liquid chromatography, part I: analytical techniques for isolated human plasma. J Anal Toxicol 1980;4:12–5.
Akpofure C, Riley CA, Sinkule JA, Evans WE. Quantitation of daunorubicin and its metabolites by high-performance liquid chromatography with electrochemical detection. J Chromatogr 1982;232:377–83.
Kotake AN, Vogelzang NJ, Larson RA, Choporis N. New high-performance liquid Chromatographic assay for plasma doxorubicin. J Chromatogr 1985;337: 194–200.
Fandrich S, Pittman KA. Analysis of carminomycin in human serum by fluorometric high-performance liquid chromatography. J Chromatogr 1981;223:155–64.
Moro E, Bellotti V, Stegnjaich S, Valzelli G. Assay of metabolites of epirubicin by HPLC in human serum. 9th International Symposium on Column Chromatography, Edinburgh, July, 1985;P02.45:97.
Cummings J, Stuart JFB, McArdle CS, Caiman KC. Identification of adriamycin and its metabolites in human and animal tissue and blood. Methodol Surv Biochem Anal 1984;14:245–6.
Gray PJ, Phillips DR. Ultraviolet photoirradiation of daunomycin and daunomycin complexes. Photochem Photobiol 1981;33:297–303.
Scourides PA, Brownlee RTC, Phillips DR, Reiss JA. Application of analytical and semi-preparative highperformance liquid chromatography to anthracyclines and bis-anthracycline derivatives. J Chromatogr 1984; 288:127–36.
Strauss JF, Kitchens RL, Patrizi VW, Frenkel EP. Extraction and quantification of daunomycin and doxorubicin in tissues. J Chromatogr 1980;221:139–44.
El-Yazigi A, Al-Saleh I. Rapid analysis of doxorubicin in plasma by radial compression liquid chromatography. J Pharm Sci 1985;74:1225–7.
Erttmann R. Determination of aclacinomycin A by reversed-phase high-performance liquid chromatography. J Chromatogr 1983;277:433–5.
Peters JH, Gordon GR, Kashiwase D, Acton EM. Metabolic disposition of 5-iminodaunorubicin in the rat. Cancer Res 1984;44:1453–9.
Janssen MJH, Crommelin DJA, Storm G, Hulshoff A. Doxorubicin decomposition on storage. Effect of pH, type of buffer and liposome encapsulation. Int J Pharm 1985;23:1–11.
White ER, Zarembo JE. Reversed-phase high speed liquid chromatography of antibiotics, III. Use of ultra high performance columns and ion-pairing techniques. J Antibiot 1981;34:836–44.
Van Lancker MA, Nelis HJCF, De Leenheer AP. Reversed-phase ion pair chromatography of anthracyclines. J Chromatogr 1983;254:45–52.
Thomas AH, Quinlan GJ, Gutteridge JMC. Assay of doxorubicin and 4′-epidoxorubicin by reversed phase ion-pair chromatography. J Chromatogr 1984;299: 489–94.
Pratt EA, McGovren JP, Adams WJ, Hamilton RD. Bioavailability and pharmacokinetics of orally administered 7-con-O-methylnogaroi (7-OMEN; NSC 269148) in the mouse. Proc Am Assoc Cancer Res 1982; 23:171.
McGovren JP, Hamilton RD, Adams WJ, Pratt EA. Quantitation of anthracycline antitumor agent menogarol in plasma using liquid chromatography with fluorescence detection. Anal Chem 1984;56:1587–90.
Dodion P, Chang BK, Egorin MJ, Olman EA, Engisch KL, Bachur NR. The disposition of the new anthracycline antibiotic, menogarol, in mice. Drug Metab Dispos 1984;12:365–70.
Fujiwara A, Hoshino T, Tazoe M, Fujiwara M. Auromycins and sulfurmycins, new anthracycline antibiotics: characterization of aglycones, auramycinone, sulfurmycinone. J Antibiot 1981;34:608–10.
Hoffman DM, Grossano DD, Damin LA, Woodcock TM. Stability of refrigerated and frozen solutions of doxorubicin hydrochloride. Am J Hosp Pharm 1979; 36:1536–8.
Camaggi CM, Strocchi E, Tamassia V, et al. Pharmacokinetic studies of 4′-epidoxorubicin in cancer patients with normal and impaired renal function and with hepatic metastases. Cancer Treat Rep 1982;66:1819–24.
Watson ID, Stewart MJ, Farid YYZ. The effect of surfactants on the high-performance liquid chromatography of anthracyclines. J Pharm Biomed Anal 1985;3:555–63.
Deesen PE, Leyland-Jones B. Sensitive and specific determination of the new anthracycline analog 4′-epidoxorubicin and its metabolites by high pressure liquid chromatography. Drug Metab Dispos 1984;12: 9–13.
Parker RJ, Priester ER, Sieber SM. Effect of route administration and liposome entrapment on the metabolism and disposition of adriamycin in the rat. Drug Metab Dispos 1982;10:499–504.
Daghestani AN, Arlin ZA, Leyland-Jones B, et al. Phase I and II clinical and pharmacological study of 4-demethoxydaunorubicin (Idarubicin) in adult patients with acute leukemia. Cancer Res 1985;45: 1408–12.
Langone J, Van Vunakis H, Bachur NR. Adriamycin and metabolites: Separation by high-pressure liquid chromatography and quantitation by radioimmunoassay. Biochem Med 1975;12:283–9.
Leyland-Jones B, Deesen P, Wittes R, Young CW. High-pressure liquid chromatography methodology and human plasma pharmacokinetics of 4′-epidoxorubicin and its metabolites. Clin Pharmacol Ther 1982; 33:244.
Imamura K, Odagawa A, Tanabe K, Hayakawa Y, Otake N. Akrobomycin, a new anthracycline antibiotic. J Antibiot 1984;37:83–4.
Uchida T, Imoto M, Masuda T, et al. New anthracycline antibiotics: serirubicin and I-hydroxyserirubicin. J Antibiot 1985;38:795–8.
Arcamone F, Cassinelli G, Franceschi G, Mondelli R, Orezzi P, Penco S. Struttura e stereochimica della daunomicina. Gazz Chim Ital 1970;100:949–89.
Kaniewska T. Badanie rozkladu adriamycyny. Farmacja Polska 1977;33:539–42.
Winchester JF, Rahman A, Tilstone WJ, et al. Sorbent removal of adriamycinin vitro andin vivo. Cancer Treat Rep 1979;63:1787–93.
Daugherty JP, Hixon SC. Photolytic destruction of adriamycin. J Pharm Pharmacol 1981;33:556.
Tavoloni N, Guraino AM, Berk PD. Photolytic degradation of adriamycin. J Pharm Pharmacol 1980;32: 860–2.
Thoma K, Strittmatter T, Steinbach D. Untersuchungen zur Photoinstabilität von Antibiotika. Acta Pharm Tech 1980;26:269–72.
Daugherty JP, Hixon SC, Yielding KL. Directin vitro photoaffinity labeling of DNA with daunorubicin, adriamycin and rubidazone. Biochim Biophys Acta 1979;565:13–21.
Williams BA, Tritton TR. Photoinactivation of anthracyclines. Photochem Photobiol 1981;34:131–4.
Anne A, Moiroux J. One-electron and two-electron reductions of daunomycin. Nouv J Chim 1985;9:83–9.
Higuchi T, Kotwal PM. Complexes of doxorubicin exhibiting enhanced stability. US Patent 1981: 4,246,399.
Beijnen JH, Van der Houwen OAGJ, Voskuilen MCH, Underberg WJM. Aspects of the degradation kinetics of daunorubicin in aqueous solution. Int J Pharm (in press).
Projan A, Nissen E. Beitrag zur Überprüfung der Stabilität von Daunoblastinlösungen. Arch Geschwulstforsch 1976;46:50–7.
Yang LY, Drewinko B. Cytotoxic efficacy of reconstituted and stored antitumor agents. Cancer Res 1985; 45:1511–5.
Hildebrand-Zanki SU, Kern DH. A new bioassay forin vitro drug stability. In: Salmon SE, Trent JM, eds. Human tumor cloning. New York: Grune and Stratton, 1984:451–8.
Ketchum D, Wolf E, Sesin GP. Cost benefit and stability study of doxorubicin following reconstitution. Am J Intrav Ther Clin Nutr 1981:15–8.
Arcamone F, Franceschi G, Penco S. Process for the preparation of adriamycin and adriamycinone and adriamycin derivatives. US Patent 1974:3,803,124.
US Pharmacopeia. Rockville: The United States Pharmacopeial Convention Inc., 1985:274, 357.
Beijnen JH, Rosing H, De Vries PhA, Underberg WJM. Stability of anthracycline antitumour agents in infusion fluids. J Parent Sci Technol 1985;39:220–2.
Yanagawa C, Tanaka Y, Fukuda M. Stability and releasability of adriamycin ointment. Byoin Yakugaku 1983;9:394–7.
Cofrancesco E, Vigo A, Pogliani E. Antiheparin activity of adriamycin. Thromb Res 1980;18:743–6.
Broggini M, Colombo T, Garattini S, Donelli MG. Influence of tumor on adriamycin concentration in blood cells. Cancer Chemother Pharmacol 1980;4: 209–12.
Eksborg S, Ehrsson H, Wallin I, Lindfors A. Quantitative determination of adriamycin and daunorubicin — handling of blood and plasma samples. Acta Pharm Suec 1981;18:215–20.
Huffman DH, Bachur NR. Daunorubicin metabolism by human hematological components. Cancer Res 1972;32:600–5.
Johansen PB, Jensen SE, Rasmussen SN, Dalmark M. Pharmacokinetics of doxorubicin and its metabolite doxorubicinol in rabbits with induced acid and alkaline urine. Cancer Chemother Pharmacol 1984; 13:5–8.
Slee PHThJ, Van Oosterom AT, De Bruijn EA. Predictive testing in cancer chemotherapy. II.In vitro. Pharm Weekbl [Sci] 1985;7:125–33.
Ludwig R, Alberts DS. Chemical and biological stability of anticancer drugs used in a human tumor clonogenic assay. Cancer Chemother Pharmacol 1984;12: 142–5.
Pavlik EJ, Kenady DE, Van Nagell JR, et al. Stability of doxorubicin in relation to chemosensitivity determinations: loss of lethality and relation of antiproliferative activity. Cancer Invest 1984;2:449–58.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bouma, J., Beijnen, J.H., Bult, A. et al. Anthracycline antitumour agents. Pharmaceutisch Weekblad Scientific Edition 8, 109–133 (1986). https://doi.org/10.1007/BF02086146
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02086146