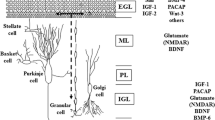
Summary
Cultures of dissociated cerebellum from 5- to 6-day-old mice as well as of the N2A neuronal cell line were exposed to guanidino ethane sulfonate (GES, 2–5 mM) to reduce the cellular taurine content. Control cultures were kept in culture medium or medium containing 2–5 mM GES plus 2–5 mM taurine to restore the intracellular taurine content. Taurine depletion led to changes in the expression of certain splice variants of NCAM mRNA such as the AAG and the VASE containing forms, while no differences were seen in the expression of the three forms of NCAM protein. In the N2A cells taurine depletion led to a decreased migration rate of the cells. The results suggest that the reduced migration rate of neurons caused by taurine depletion may be correlated to changes in expression of certain adhesion molecules such as NCAM. Moreover, taurine appears to be involved in regulation of transcription processes.
Similar content being viewed by others
References
Andersson AM, Gaardsvoll H, Giladi E, Dahl B, Bock E (1990) Characterization of rat brain NCAM mRNA using DNA oligonucleotide probes. FEBS Lett 263: 385–388
Burg MB, Kwon ED, Kultz D (1997) Regulation of gene expression by hypertonicity. Annu Rev Physiol 59: 437–455
Chen A, Haines S, Maxson K, Akeson RA (1994) VASE exon expression alters NCAM-mediated cell-cell interactions. J Neurosci Res 38: 483–492
Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kramer P, Scheff S, Barthels D, Rajewsky K, Wille W (1994) Inactivation of the NCAM gene in mice results in reduction of the olfactory bulb and deficits in spatial learning. Nature 367: 455–459
Doherty P, Moolenaar CECK, Ashton SV, Michalides RJAM, Walsh ITS (1992) Use of the VASE exon downregulates the neurite growth promoting activity of NCAM 140. Nature 356: 791–793
Doherty P, Smith P, Walsh FS (1996) Shared cell adhesion molecule (CAM) homology domains point to CAMs signalling via FGF receptors. Perspect Dev Neurobiol 4: 157–168
Edmondson JC, Liem RKH, Kuster JE, Hatten ME (1988) Astrotactin: a novel neuronal cell surface antigen that mediates neuron-astroglial interactions in cerebellar microcultures. J Cell Biol 106: 505–517
Fuhrman S, Kirsch M, Hofmann HD (1995) Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development 121: 2695–2706
Gegelashvili G, Bock E (1996) Cell recognition molecules of immunoglobulin superfamily in the nervous system. In: Lee AG (ed) Biomembranes, vol 3. JAI Press Inc, Greenwich, London, pp 33–75
Gegelashvili G, Andersson A-M, Schousboe A, Bock E (1993a) Characterization of NCAM diversity in cultured neurons. FEBS Lett 324: 337–340
Gegelashvili G, Andersson A-M, Schousboe A, Bock E (1993b) Cyclic AMP regulates NCAM expression and phosphorylation in cultured mouse astrocytes. Eur J Cell Biol 62: 343–351
Hatten ME, Mason CA (1990) Mechanisms of glial-guided neuronal migrationin vitro andin vivo. Experientia 46: 907–916
Hayes KC, Carey RE, Schmidt SY (1975) Reginal degeneration associated with taurine deficiency in the cat. Science 188: 949–951
Huxtable RJ, Laird HE, Lippincott SE (1979) The transport of taurine in the heart and the rapid depletion of tissue taurine content by guanidino ethane sulfonate. J Pharmacol Exp Ther 211: 465–471
Inoue M, Nakayama C, Noguchi H (1996) Activating mechanism for CNTF and related cytokines. Mol Neurobiol 12: 195–209
Itoh T, Yamauchi A, Miyai A. Yokoyama K, Kamada T, Ueda N, Fujiiwara Y (1994) Mitogen-activated protein kinase and its activator are regulated by hypertonic stress in Madin-Darby canine kidney cells. J Clin Invest 93:2387–2392
Lindner J, Rathjen FG, Schachner M (1983) L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature 305: 427–430
Lombardini JB (1995) Paradoxical stimulatory effect of the kinase inhibitor chelerythrine on the phosphorylation of a approximately 20K Mr protein present in the mitochondrial fraction of rat retina. Brain Res 673: 194–198
Lombardini JB, Props C (1996) Effects of kinase inhibitors and taurine analogues on the phosphorylation of specific proteins in mitochondrial fractions of rat heart and retina. Adv Exp Med Biol 403: 343–350
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275
Lyons GE, Moore R, Yahara O, Buckingham ME, Walsh FS (1992) Expression of NCAM isoforms during skeletal myogenesis in the mouse embryo. Dev Dynamics 194: 94–104
Moran J, Pasantes-Morales H (1991) Taurine-deficient cultured cerebellar astrocytes and granule neurons obtained by treatment with guanidino ethane sulfonate. J Neurosci Res 29: 533–537
Moran J, Maar T, Gegelashvili G, Bock E, Schousboe A, Pasantes-Morales H (1996) Taurine deficiency and neuronal migration. Adv Exp Med Biol 403: 519–526
Maar T, Moran J, Schousboe A, Pasantes-Morales H (1995) Taurine deficiency in dissociated mouse cerebellar cultures affects neuronal migration. Int J Devl Neuroscience 13: 491–502
Maar TE, Rønn LCB, Bock E, Berezin V, Moran J, Pasantes-Morales H, Schousboe A (1997) Characterization of microwell cultures of dissociated brain tissue for studies of cell-cell interactions. J Neurosci Res 47: 163–172
Ono K, Tomasiewitz H, Magnussen T, Rutishauser U (1994) N-CAM mutation inhibits tangential neuronal migration and is phenocopied by enzymatic removal of polysialic acid. Neuron 13: 595–609
Pasantes-Morales H, Schousboe A (1997) Role of taurine in osmoregulation in brain cells: mechanisms and functional implications. Amino Acids 12: 281–292
Persohn E, Schachner M (1987) Immunoelectron microscopic localization of the neural cell adhesion molecules L1 and N-CAM during postnatal development of the mouse cerebellum. J Cell Biol 105: 569–576
Rakic P (1971) Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electron microscopy study inMacacus rhesus. J Comp Neurol 141: 283–312
Rakic P, Cameron RS, Komuro H (1994) Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol 4: 6369
Schousboe A, Meier E, Drejer J, Hertz L (1989) Preparation of primary cultures of mouse (rat) cerebellar granule cells. In: Shahar A, De Vellis J, Vernadakis A, Haber B (eds) A dissection and tissue culture manual of the nervous system. Alan R Liss, New York, pp 203–206
Son M, Kim HK, Kim WB, Yang J, Kim BK (1996) Protective effect of taurine on indomethacin-induced gastric mucosal injury. Adv Exp Med Biol 403: 147–155
Sturman JA, Moretz RC, French JH, Wiesniewski HM (1985a) Taurine deficiency in the developing cat: persistence of the cerebellar granule cell layer. J Neurosci Res 13: 405–416
Sturman JA, Moretz RC, French JH, Wiesniewski HM (1985b) Postnatal taurine deficiency in the kitten results in a persistence of the cerebellar external granule cell layer: correction by taurine feeding. J Neurosci Res 13: 521–528
Tomaselli KJ, Reichardt LF, Bixby JL (1986) Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol 103: 2659–2672
Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF (1988) N-Cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron 1: 33–43
Trenkner E, Sidman RL (1977) Histogenesis of mouse cerebellum in microwell cultures, cell reaggregation and migration, fiber and synapse formation. J Cell Biol 75: 915–940
Trenkner E, el-Idrissi A, Harris C (1996) Balanced interaction of growth factors and taurine regulates energy metabolism, neuronal survival, and function of cultured mouse cerebellar cells under depolarizing conditions. Adv Exp Med Biol 403: 507–517
Walsh FS, Doherty P (1991) Structure and function of the gene for neural cell adhesion molecule. Semin Neurosci 3: 271–284
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maar, T.E., Lund, T.M., Gegelashvili, G. et al. Effects of taurine depletion on cell migration and NCAM expression in cultures of dissociated mouse cerebellum and N2A cells. Amino Acids 15, 77–88 (1998). https://doi.org/10.1007/BF01345281
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01345281