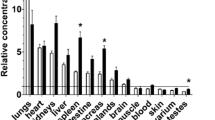
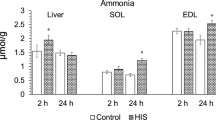
Summary
This study followed the time course of urinary taurine and hypotaurine excretion after two-thirds hepatectomy in rats. The excretion of both taurine and hypotaurine was elevated during 18th following the hepatectomy, with maximal excretion during the first 6h. Twelve and 24h after partial hepatectomy, the hepatic hypotaurine concentration was increased but liver taurine did not differ significantly from controls. No changes were observed in hypotaurine and taurine concentrations of heart, kidney, lung, muscle tissue and spleen. We postulate that partial hepatectomy induces a rapid increase of hepatic (hypo)taurine synthesis from precursor amino acids. The increased (hypo)taurine concentrations spill over into urine.
Similar content being viewed by others
References
Akaza K, Nonami T, Kurokawa T, et al (1996) Doxorubicin-induced disturbance of the energy metabolism after hepatectomy. J Surg Res 61: 454–458
Brand HS, Meijer AJ, Gustafson LA, et al (1994) Cell-swelling-induced taurine release from isolated perfused rat liver. Biochem Cell Biol 72: 8–11
Brand HS, Deutz NEP, Meijer AJ, Jörning GGA, Chamuleau RAFM (1995a) In vivo amino acid fluxes in regenerating liver after two-thirds hepatectomy in the rat. J Hepatol 23: 333–340
Brand HS, Meijer AJ, Gustafson LA, Chamuleau RAFM (1995b) Hypoosmotic cell-swelling induced taurine release from rat liver. In: Capocaccia L, Merli M, Riggio O (eds) Advances in hepatic encephalopathy and metabolic nitrogen exchange. CRC Press, Boca Raton, pp 500–506
Le Cam A, Rey J-F, Fehlmann M, Kitabgi P, Freychet P (1979) Amino acid transport in isolated hepatocytes after partial hepatectomy in the rat. Am J Physiol 236: E594-E602
Chesney RW (1985) Taurine: its biological role and clinical implications. Adv Pediatr 32: 1–42
Clawson GA, Madsen KR, Blankenship LJ, Hatem CL (1991) Alterations in nuclear scaffold constituents during carbon tetrachloride-induced liver regeneration. Hepatology 13: 515–522
Cockerill MJ, Player TJ, Horton AA (1983) Studies on lipid peroxidation in regenerating rat liver. Biochim Biophys Acta 750: 208–213
Dejong CHC, Kampman MT, Deutz NEP, Soeters PB (1992) Altered glutamine metabolism in rat portal drained viscera and hindquarter during hyperammonemia. Gastroenterology 102: 936–948
Van Eijk HMH, van der Heijden MAH, van Berlo CLH, Soeters PB (1988) Fully automated liquid-chromatographic determination of amino acids. Clin Chem 34: 2510–2513
Ensunsa JL, Hirschberger LL, Stipanuk MH (1993) Catabolism of cystein, cystine, cysteinesulfmate and OTC by isolated perfused rat hindquarter. Am J Physiol 264: E782-E789
Fleck C, Zimmermann T, Franke H, Braunlich H, Dargel R (1988) Relation between renal and hepatic excretion of drugs: VII. Hepatic and renal excretion of phenol red in thioacetamide-induced acute and chronic liver damage. Exp Pathol 33: 47–54
Fowler FC, Banks RK, Mailliard ME (1992) Characterization of sodium-dependent amino acid transport activity during liver regeneration. Hepatology 16: 1187–1194
Garcia RAG, Stipanuk MH (1992) The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122: 1693–1701
Garcia-Marin JJ, Regueiro P, Perez-Antona JC, Villanueva GR, Perez-Barriocanal (1990) Pre-replicative phase-related changes in bile acid-induced chloleresis in the regenerating rat liver. Clin Sci 78: 55–62
Gaull GE (1989) Taurine in pediatric nutrition. Review and update. Pediatrics 83: 433–442
Grisham JW, Tillman RL, Nägel AEH, Compagno J (1975) Ultrastructure of the proliferating hepatocyte: sinusoidal surfaces and endoplasmic reticulum. In: Lesch R, Reutter W (eds) Liver regeneration after experimental injury. Stratton, New York, pp 6–23
Heeneman S, Deutz NEP (1993) Effects of decreased glutamine supply on gut and liver metabolism in vivo in rats. Clin Sci 85: 437–444
Higgins GM, Anderson RM (1931) Restoration of the liver of white rat following partial surgical removal. Arch Pathol 12: 186–202
Holtta E, Sinervirta R, Janne J (1973) Synthesis and accumulation of polyamines in rat liver regenerating after treatment with carbon tetrachloride. Biochem Biophys Res Comm 54: 350–357
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72: 101–163
Lorenzi M, De Martino A, Carlucci F, et al (1993) Nitrogen metabolism during liver regeneration. Biochim Biophys Acta 1157: 9–14
Luk GD (1986) Essential role of polyamine metabolism in hepatic regeneration. Inhibition of deoxyribonucleic acid and protein synthesis and tissue regeneration by difluoromethylornithine in the rat. Gastroenterology 90: 1261–1267
Margeli AP, Theocharis SE, Yannacou NN, Spiliopoulou C (1994) Metallothionein expression during liver regeneration after partial hepatectomy in cadmium-pretreated rats. Arch Toxicol 68: 637–642
Martinez-Mas J-V, Ruiz-Montasell B, Felipe A, Casado J, Pastor-Anglada M (1993) Up-regulation of system A activity in the regenerating rat liver. FEBS Lett 329: 189–193
Meijer AJ, Lamers WH, Chamuleau RAFM (1990) Nitrogen metabolism and ornithine cycle function. Physiol Rec 70: 701–748
Murawaki Y, Ikuta Y, Yamamoto H, Kawasaki H (1992) Serum amino acid levels and hepatic protein synthesis during liver regeneration after partial hepatectomy in rats. Res Comm Chem Pathol Pharmacol 77: 43–54
Nakata R, Tsukamoto I, Miyoshi M, Kojo S (1985) Liver regeneration after carbon tetrachloride intoxication in the rat. Biochem Pharmacol 34: 586–588
Ogawa M, Mori T, Mori Y, et al (1992) Study on chronic renal injuries induced by carbon tetrachloride: selective inhibition of the nephrotoxicity by irradiation. Nephron 60: 68–73
Ohno T, Sabra R, Branch RA (1991) Sodium retention and hepatic function after two-thirds hepatectomy in the rat. Hepatology 14: 511–517
Okano K, Tsubouchi T, Yamashita Y, et al (1997) Hepatic protein synthesis in the regenerating rat liver after hepatectopancreatectomy. Surg Today Jpn J Surg 27: 511–517
Pasantes-Morales H, Chatagner F, Mandel P (1980) Synthesis of taurine in rat liver and brain in vivo. Neurochem Res 5: 441–451
Perez-Barriocanal F, Perez-Antona JC, Regueiro P, Villanueva GR, Marin JJG (1990) Biliary lipid secretion during the pre-replicative phase of rat liver regeneration. J Exp Path 71: 63–68
Ryoo HY, Taga M, Sassa T, Oka T, Natori Y (1997) Endocytosis of serum albumin in regenerating liver. Proc Soc Exp Med Biol 215: 179–185
Sainz GR, Monte MJ, Barbero ER, Herrera MC, Marin JJG (1997) Bile secretion by the rat liver during synchronized regeneration. Int J Exp Path 78: 109–116
Sanins SM, Nicholson JK, Elcombe C, Timbrell JA (1990) Hepatotoxin-induced hypertaurinuria: a proton NMR study. Arch Toxicol 64: 407–411
Sasaki Y, Hayashi N, Ito T, Fusamoto H, Sato N, Kamada T (1989) Heterogeneous activation of protein kinase C during rat liver regeneration induced by carbon tetrachloride administration. FEBS Lett 254: 59–65
Scornik OA (1974) In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem 249: 3876–3883
Sharma R, Kodavanti UP, Smith LL, Mehendale HM (1995) The uptake and metabolism of cytamine and taurine by isolated perfused rat and rabbit lungs. Int J Biochem Cell Biol 27: 655–664
Sturman JA (1980) Formation and accumulation of hypotaurine in rat liver after partial hepatectomy. Life Sci 26: 267–272
Sturman JA, Fellman JH (1982) Taurine metabolism in the rat: effect of partial hepatectomy. Int J Biochem 14: 1055–1060
Sturman JA, Fellman JH (1983) Methionine metabolism in the rat: accumulation of hypotaurine after partial hepatectomy. Progr Clin Biol Res 125: 435–447
Tamura J, Ohkuma S, Ida S, Zuo PP, Kuriyama K (1984) Cysteine uptake and taurine biosynthesis in freshly isolated and primary cultured rat hepatocytes. Cell Biochem Funct 2: 195–200
Teshigawara M, Matsumoto S, Tsuboi S, Ohmori S (1995) Changes in levels of glutathione and related compunds and activities of glutathione-related enzymes during rat liver regeneration. Res Exp Med 195: 55–60
Timbrell JA, Waterfield CJ (1996) Changes in taurine as an indicator of hepatic dysfunction and biochemical perturbations. Adv Exp Med Biol 403: 125–134
Timbrell JA, Waterfield CJ, Draper RP (1995) Use of urinary taurine and creatine as biomarkers of organ dysfunction and metabolic perturbations. Comp Haematol Int 5: 112–119
Tohyama C, Suzuki JS, Hemelraad J, Nishimura N, Nishimura H (1993) Induction of metallothionein and its localization in the nucleus of rat hepatocytes after partial hepatectomy. Hepatology 18: 1193–1201
Tsuboi S, Miyazaki M, Kondo Y, et al (1992) Increase of S-(1,2-dicarboxyethyl)glutathione in regenerating rat liver. Res Exp Med 192: 281–286
Tsujikawa K, Suzuki N, Sagawa K, et al (1994) Induction and subcellular localization of metallothionein in regenerating rat liver. Eur J Cell Biol 63: 240–246
Waterfield CJ, Turton JA, Scales MDC, Timbrell JA (1991) Taurine, a possible urinary marker of liver damage: a study of taurien excretion in carbon tetrachloride-treated rats. Arch Toxicol 65: 548–555
Waterfield CJ, Turton JA, Scales MDC, Timbrell JA (1993a) Investigations into the effects of various hepatotoxic compounds on urinary and liver taurine levels in rats. Arch Toxicol 67: 244–254
Waterfield CJ, Turton JA, Scales MD, Timbrell JA (1993b) Effect of various non-hepatotoxic compounds on urinary and liver taurine levels in rats. Arch Toxicol 67: 538–546
Waterfield CJ, Turton JA, Scales MDC, Timbrell JA (1993c) Reduction of liver taurine in rats by β-alanine treatmment increases carbon tetrachloride toxicity. Toxicology 77: 7–20
Waterfield CJ, Asker DS, Timbrell JA (1996) Does urinary taurine reflect changes in protein metabolism? a study with cycloheximide in rats. Biomarkers 1: 107–114
Wollenberger A, Ristau O, Schoffa G (1960) Eine einfache Technik der extrem schnellen Abkählung grösserer Gewebestücke. Pflügers Arch 270: 399–412
Wright CE, Tallan HH, Lin YY, Gaul GE (1986) Taurine — biological update. Annu Rev Biochem 55: 427–453
Zimmermann SW, Norback DH, Powers K (1983) Carbon tetrachloride nephrotoxicity in rats with reduced renal mass. Arch Pathol Lab Med 107: 264–269
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brand, H.S., Jörning, G.G.A. & Chamuleau, R.A.F.M. Changes in urinary taurine and hypotaurine excretion after two-thirds hepatectomy in the rat. Amino Acids 15, 373–383 (1998). https://doi.org/10.1007/BF01320901
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01320901