Summary
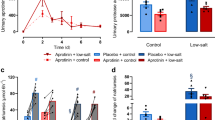
In anaesthetized adult female rats, the influence of epidermal growth factor (EGF) on renal amino acid handling was investigated in glutamine, arginine (both 50 mg/100 g b. wt. per hour), or alanine (90 mg/ 100 g b. wt. per hour) loaded animals. Continuous infusions of the three amino acids were followed by an increase in the fractional excretion (FE) of the administered amino acids as well as of the other endogenous amino acids. Under load conditions (alanine, arginine or glutamine), EGF pretreatment (8μg/100g b. wt. subcutaneously for 8 days, twice daily 8 a.m. and 4 p.m.) was followed by a stimulation of renal amino acid reabsorption. The increase in the fractional excretion of the administered amino acids was significantly lower than in non-EGF-treated rats. These changes in amino acid transport were connected with a significant reduction of GFR after EGF pretreatment (0.96 ± 0.10 vs. 0.62 ± 0.07 ml/min X 100 g b. wt.) and a distinct increase in sodium excretion (2.98 ± 0.55 vs. 4.97 ± 0.71μval/100 g b. wt. X 20 min). After loading with p-aminohippurate (PAH; 200mg/100g b. wt.), PAH excretion in EGF rats was increased by about 20%, whereas urinary protein excretion was lower in EGF pretreated rats (control: 0.45 ± 0.04 vs. EGF: 0.18 ± 0.03 mg/ 100 g b. wt. X 20 min). The PAH load reduced amino acid reabsorption as a sign of overloading of renal tubular transport capacity, but in EGF pretreated animals the amino acid excretion was only slightly increased under these conditions. Furthermore, EGF pretreatment depressed normal kidney weight gain significantly (874 ± 18 vs. 775 ± 32mg/100g b. wt.). EGF can improve the renal tubular transport capacity, but, compared to well-known stimulators of renal transport like dexamethasone or tri-iodothyronine, its effect is only of a moderate degree.
Similar content being viewed by others
References
Boerner P, Resnick RJ, Racker E (1985) Stimulation of glycolysis and amino acid uptake in NRK-49F cells by transforming growth factor beta and epidermal growth factor. Proc Natl Acad Sci USA 82: 1350–1353
Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, Henegouwen PVE (1995) The epidermal growth factor. Cell Biol Internat 19: 413–430
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Bratton AC, Marshall EK (1939) A new coupling component for sulfonamide determination. J Biol Chem 128: 537–550
Bräunlich H, Fleck C, Bajanowski T, Miosge W (1983) Dosisabhdngigkeit und Altersabhdngigkeit des renalen tubulären Transportes von p-Aminohippursäure (PAH) bei Ratten nach Injektion von Einzeldosen. Pharmazie 38: 483–485
Bräunlich, H (1984) Postnatal development of kidney function in rats receiving thyroid hormones. Exp Clin Endokrinol 83: 243–250
Breyer MD, Jacobson HR, Breyer JA (1988) Epidermal growth factor inhibits the hydroosmotic effect of vasopressin in the isolated perfused rabbit cortical collecting tubule. J Clin Invest 82: 1313–1320
Carpenter G, Cohen S (1990) Epidermal growth factor. J Biol Chem 265: 7709–7712
Christensen HN (1990) Role of amino acid transport and counter-transport in nutrition and metabolism. Physiol Rev 70: 43–71
Fernandez-Pol JA, Klos DJ, Hamilton PD (1989a) Modulation of transforming growth factor alpha-dependent expression of epidermal growth factor receptor gene by transforming growth factor beta, triiodothyronine, and retinoic acid. J Cell Biochem 41: 159–170
Fernandez-Pol JA, Hamilton PD, Klos DJ (1989b) Transcriptional regulation of protooncogene expression by epidermal growth factor, transforming growth factor beta 1, and triiodothyronine in MDA-468 cells. J Biol Chem 264: 4151–4156
Fisher DA, Salido EC, Barajas L (1989) Epidermal growth factor and the kidney. Ann Rev Physiol 51: 67–80
Fleck C, Bräunlich H (1986) Relation between renal and hepatic excretion of drugs: I Phenol red in comparison with p-aminohippurate and indocyanine green. Exp Pathol 29: 179–192
Fleck C, Börner A, Kretzschmar M, Machnik G, Sprott H, Zimmermann T, Keil E, Bräunlich H (1992) Liver function after bilateral nephrectomy. Liver 12: 319–325
Fleck C, Aurich M, Schwertfeger M (1997) Stimulation of renal amino acid reabsorption after treatment with triiodothyronine or dexamethasone in amino acid loaded rats. Amino Acids 12: 265–279
Gow CB, Phillips PA (1994) Epidermal growth factor as a diuretic in sheep. J Physiol 477: 27–33
Gresik EW, Maruyama S (1987) Inductive effects of triiodothyronine or dihydrotestosterone on EGF in the submandibular glands of young, middle-aged, and old C57BL/6NNia female mice. J Gerontol 42: 491–496
Gutmann M, Hoischen C, Krämer R (1993) Carrier mediated glutamate secretion by corynebacterium glutamicum under biotin limitation. Biochim Biophys Acta 1112: 115–123
Harris RC (1991) Potential physiologic roles for epidermal growth factor in the kidney. Am J Kidney Dis 17: 627–630
Harris RC, Daniel TO (1989) Epidermal growth factor binding, stimulation of phosphorylation, and inhibition of gluconeogenesis in rat proximal tubule. J Cell Physiol 139: 383–391
Incerpi S, Spagnuolo S, Terenzi F, Leoni S (1996) EGF modulation of Na+/H+ antiport in rat hepatocytes: different sensitivity in adult and fetal cells. Am J Physiol 39: C841-C847
Karasik A, Kahn CR (1988) Dexamethasone-induced changes in phosphorylation of the insulin and epidermal growth factor receptors and their substrates in intact rat hepatocytes. Endocrinology 123: 2214–2222
Keiser JA, Ryan MJ (1996) Hemodynamic effects of epidermal growth factor in conscious rats and monkeys. Proc Natl Acad Sci USA 93: 4957–4961
Konturek SJ, Pawlik W, Mysh W, Gustaw P, Sendur R, Mikos E, Bielanski W (1990) Comparison of organ uptake and disappearance half-time of human epidermal growth factor and insulin. Regul Pept 30: 137–148
MacDougall ML, Wiegmann TB (1988) Excretion of para-aminohippurate in the isolated perfused rat kidney: net secretion and net reabsorption. J Physiol Lond 397: 459–469
Moffatt P, Plaa GL, Denizeau F (1995) Induction of metallothionein gene expression by epidermal growth factor and its inhibition by transforming growth factor-beta and dexamethasone in rat hepatocytes. Hepatology 21: 1038–1044
Nielsen S, Nexo E, Christensen El (1989) Absorption of epidermal growth factor and insulin in rabbit renal proximal tubules. Am J Physiol 256: E55-E63
Norman J, Tsau Y-K, Bacay A, Fine LG (1990) Epidermal growth factor accelerates functional recovery from ischaemic acute tubular necrosis in the rat: role of the epidermal growth factor receptor. Clin Sci 78: 445–450
Oberg KC, Carpenter G (1989) Dexamethasone acts as a negative regulator of epidermal growth factor receptor synthesis in fetal rat lung cells. Mol Endocrinol 3: 915–922
Oberg KC, Carpenter G (1991) Dexamethasone and retinoic acid regulate the expression of epidermal growth factor receptor mRNA by distinct mechanisms. J Cell Physiol 149: 244–251
Parys JB, De Smedt H, Van Den Bosch L, Geuns J, Borghgraef R (1990) Regulation of the Na(+)-dependent and the Na(+) -independent polyamine transporters in renal epithelial cells (LLC-PK1). J Cell Physiol 144: 365–375
Quigley R, Kennerly DA, Shen JN, Baum M (1995) Stimulation of proximal convoluted tubule phosphate transport by epidermal growth factor: signal transduction. Am J Physiol 269: 17339–17344
Racusen LC, Monteil C, Sgrignoli A, Lucskay M, Marouillat S, Rhim JG, Morin JP (1997) Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. J Lab Clin Med 129: 318–329
Rogers SA, Miller SB, Hammerman MR (1995) Triiodothyronine stimulates renal epidermal growth factor expression in adult rat. Am J Physiol 268: 17218–17234
Roth M (1971) Fluorescence reaction for amino acids. Anal Chem 43: 880–882
Roth, M, Hampai A (1973) Column chromatography of amino acids with fluorescence detection. J Chromatogr 83: 353–356
Salido EC, Fisher DA, Barajas L (1986) Immunoelectron microscopy of epidermal growth factor in mouse kidney. J Ultrastruct Mol Struct Res 96: 105–113
Scott SM, Rogers C, Backstrom C (1995) Dexamthasone therapy is associated with a rise in urinary epidermal growth factor concentrations in the preterm infant. Eur J Endocrinol 132: 326–330
Silbernagl S (1983) Kinetics and localisation of tubular resorption of „acidic” amino acids. A microperfusion and free flow micropuncture study in rat kidney. Pütigers Arch Eur J Physiol 396: 218–224
Silbernagl S (1992) Amino acids and oligopeptides. In: Seldin DW, Giebisch G (eds) The kidney, vol 2. Raven Press, New York, pp 2889–2920
Sohtell M, Kalmark B, Ulfendahl H (1983) FITC-inulin as a kidney tubule marker in the rat. Acta Physiol Scand 119: 313–316
Thurau K (1975) Modification of angiotensin-mediated tubulo-glomerular feedback by extracellular volume. Kidney Int [Suppl]: S202-S207
Tuomela T, Miettinen P, Pesonen K, Viinikka L, Perheentupa J (1990) Epidermal growth factor in mice: effects of estradiol, testosterone and dexamethasone. Acta Endocrinol Copenh 123: 211–217
Vehaskari VM, Herndon J, Hamm LL (1991) Mechanism of sodium transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol 261: F896-F903
Vesey DA, Selden AC, Hodgson HJ (1993) Down regulation of epidermal growth factor receptors in liver proliferation induced by a mixture of triiodothyronine, amino acids, glucagon, and heparin (TAGH). Gut 34: 1601–1606
Wang JY, Zhang LH, Song WL (1996) Epidermal growth factor regulates intestinal glutamine uptake during total parenteral nutrition. Clinical Nutrition 15: 21–23
Warden DH, Stokes JB (1993) EGF and PGE2 inhibit rabbit CCD Na+ transport by different mechanisms: PGE2 inhibits Na(+)-K+ pump. Am J Physiol 264: F670-F677
Yamamoto H, Kato Y (1992) Effects of age, sex and renal function on urinary insulin-like growth factor I (IGF-I) levels in adults. Endocrinology 39: 507–516
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fleck, C., Pertsch, J. Epidermal growth factor (EGF) increases the renal amino acid transport capacity in amino acid loaded rats. Amino Acids 15, 307–320 (1998). https://doi.org/10.1007/BF01320896
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01320896