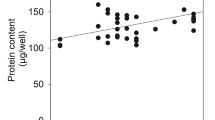
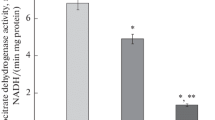
Summary
In isolated rabbit renal cortical tubules, glucose synthesis from 1 mM alanine is negligible, while the amino acid is metabolized to glutamine and glutamate. The addition of 0.5 mM octanoate plus 2 mM glycerol induces incorporation of [U-14C]Alnine into glucose and decreases glutamine synthesis, whereas oleate and palmitate in the presence of glycerol are less potent than octanoate. Gluconeogenesis is also significantly accelerated when glycerol is substituted by lactate. In view of an increase in14CO2 fixation and elevation of both cytosolic and mitochondrial NADH/NAD+ ratios, the activation of glucose formation from alanine upon the addition of glycerol and octanoate is likely due to (i) stimulation of pyruvate carboxylation, (ii) increased availability of NADH for glyceraldehyde-3-phosphate dehydrogenase and (iii) elevation of mitochondrial redox state causing a diminished provision of ammonium for glutamine synthesis. The induction of gluconeogenesis in the presence of alanine, glycerol and octanoate is not related to cell volume changes. The results presented in this paper show the importance of free fatty acids and glycerol for regulation of renal gluconeogenesis from alanine. The possible physiological significance of the data is discussed.
Similar content being viewed by others
References
Baverel G, Martin G, Michoudet Ch (1990) Glutamine synthesis from aspartate in guinea-pig renal cortex. Biochem J 268: 437–442
Beck JS, Potts DJ (1990) Cell swelling, co-transport activation and potassium conductance in isolated perfused rabbit kidney proximal tubules. J Physiol 425: 369–378
Bergmeyer HU (1965) Methods of enzymatic analysis, 2nd edn. Verlag Chemie GmbH, Weinheim; Academic Press, New York London
Berry MN, Kun E, Werner HV (1973) Regulatory role of reducing-equivalent transport from substrate to oxygen in the hepatic metabolism of glycerol and sorbitol. Eur J Biochem 33: 407–417
Berry MN, Phillips JW, Grivell AR (1992) Interactions between mitochondria and cytoplasm in isolated hepatocytes. Curr Top Cell Reg 33: 309–328
Chang J, Knecht R, Braun DG (1983) Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography. Methods Enzymol 91: 41–48
Dugelay S, Baverel G (1991) Concomitant synthesis and degradation of glutamine in isolated rabbit kidney tubules. Biochim Biophys Acta 1075: 191–194
Efthivoulou M-A, Philips JW, Berry MN (1995) Abolition of the inhibitory effect of ethanol oxidation on gluconeogenesis from lactate by asparagine or low concentrations of ammonia. Biochim Biophys Acta 1244: 303–310
Emmet M, Seldin W (1985) Metabolic acidosis and alcalosis. In: Seldin DW, Giebish G (eds) The kidney. Physiology and physiopathology. Raven Press, New York, pp 1567–1639
Exton JH, Park CR (1967) Control of gluconeogenesis in liver. J Biol Chem 242: 2622–2636
Fanelli C, Calderone S, Epifano L, De Vincenzo A, Modarelli F, Pampanelli S, Perriello G, De Feo P, Brunetti P, Gerich JE, Bolli GB (1993) Demonstration of a critical role for free fatty acids in mediating counterregulatory stimulation of gluconeogenesis and suppression of glucose utilization in humans. J Clin Invest 92: 1617–1622
Fouque D, Dugelay Y, Martin G, Combet J, Baverel G (1996) Alanine metabolism in isolated human kidney tubules. Use of a mathematical model. Eur J Biochem 236: 128–137
Friedrichs D (1975) On the stimulation of gluconeogenesis by L-lysine in isolated rat kidney cortex tubules. Biochim Biophys Acta 392: 255–270
Guder WG, Wieland OH (1972) Metabolism of isolated kidney tubules. Additive effect of parathyroid hormone and free fatty acids on renal gluconeogenesis. Eur J Biochem 31: 69–79
Harris SJ, Balaban RS, Barret L, Mandel LJ (1981) Mitochondrial respiratory capacity and Na+ - and K+ - dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem 256: 10319–10328
Häussinger D, Lang F (1991) Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta 1071: 331–350
Jungas RL, Mitchell L, Brosnan JT (1992) Quantative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev 72: 419–448
Klahr S, Hammerman M (1985) Renal metabolism. In: Seldin DW, Giebish G (eds) The kidney. Physiology and physiopathology. Raven Press, New York, pp 699–718
Lang F, Ritter M, Völkl H, Häussinger D (1993) The biological significance of cell volume. Renal Physiol Biochem 16: 48–65
LaNoue KF, Walajtys E, Williamson JR (1973) Regulation of glutamate metabolism in interactions with citric acid cycle in rat heart mitochondria. J Biol Chem 248: 7171–7183
Lemieux G, Berkofsky J, Lemieux C, Quenneville A, Marsolais M (1988) Real importance of alanine in renal metabolism: in vitro studies in dog. Am J Physiol 255: R42-R45
Lietz T, Bryla J (1995) Glycerol and lactate induce reciprocal changes in glucose formation and glutamine production in isolated rabbit kidney-cortex tubules incubated with aspartate. Arch Biochem Biophys 321: 501–509
Lietz T, Winiarska K, Bryla J (1997) Ketone bodies activate gluconeogenesis in isolated rabbit renal cortical tubules incubated with glycerol and amino acids. Acta Biochim Polon 44: 323–332
Lin ECC (1977) Glycerol utilization and its regulation in mammals. Annu Rev Biochem 46:765–795
Meister A (1984) Enzymology of glutamine. In: Häussinger D, Sies H (eds) Glutamine metabolism in mammalian tissues. Springer, Berlin Heidelberg New York Tokyo, pp 3–15
Michoudet Ch, Martin G, Baverel G (1988) Pyruvate carboxylation in glutamine synthesis from alanine by isolated guinea-pig renal cortical tubules. Pflugers Arch 412: 711
Morrand Ch, Besson C, Demigne Ch, Remesy Ch (1994) Importance of the modulation of glycolysis in the control of lactate metabolism by fatty acids in isolated hepatocytes from fed rats. Arch Biochem Biophys 309: 254–260
Niwa H, Yamano T, Sugano T (1986) Hormonal effects and control of gluconeogenesis from sorbitol, xylitol and glycerol in perfused chicken liver. Comp Biochem Physiol 8513: 739–745
Pilkis SJ, Rion JP, Claus TH (1976) Hormonal control of [14C] glucose synthesis from [14C]dihydroxyacetone and glycerol in isolated rat hepatocytes. J Biol Chem 251: 7841–7852
Prior RL, Crim MC, Castaneda C, Lammi-Keefe C, Dawson-Hughes B, Rosen CJ, Spindler AA (1996) Conditions altering plasma concentrations of urea cycle and other amino acids in erderly human subjects. J Am Coll Nutr 15: 237–247
Randle PJ (1995) Metabolic fuel selection: general integration at the whole-body level. Proc Nutr Soc 54: 317–327
Scholz R, Olson MS, Schwab AJ (1978) The effect of fatty acids on the regulation of pyruvate dehydrogenase in perfused rat liver. Eur J Biochem 86: 519–530
Scholz TD, Laughlin MR, Balaban RS, Kupriyanov VV, Heineman FW (1995) Effect of substrate on mitochondrial NADH, cytosolic redox state and phosphorylated compounds in isolated hearts. Am J Physiol 268: H82-H91
Scrutton MC, Young MR (1972) Pyruvate carboxylase. In: Boyer PD (ed) The enzymes. Academic Press, New York London, pp 1–35
Silbernagl S (1985) Amino acids and oligopeptides. In: Seldin DW, Giebish G (eds) The kidney. Physiology and physiopathology. Raven Press, New York, pp 1677–1701
Sugano T, Nishimura K, Sogabe N, Shiota M, Oyama N, Noda S, Ohta M (1988) Ca-dependent activation of the malate-aspartate shuttle by norepinephrine and vasopressin in perfused rat liver. Arch Biochem Biophys 264: 144–154
Walajtys-Rode E, Zapareto J, Moehren G, Hoek JB (1992) The role of matrix calcium in the enhancement of mitochondria) pyruvate carboxylation by glucagon pretreatment. J Biol Chem 267: 370–379
Weinberg JM, Davis JA, Abarzua M, Smith RK, Kunkel R (1990) Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol 258: F346-F355
Werner HV, Berry MN (1974) Stimulatory effect of thyroxine administration on reducing-equivalent transport from substrate to oxygen during hepatic metabolism of sorbitol and glycerol. Eur J Biochem 42: 315–324
Wettstein M, vom Dahl S, Lang F, Gerok W, Häussinger D (1990) Cell volume regulatory responses of isolated perfused rat liver. The effect of amino acids. Biol Chem Hoppe-Seyler 37:493–501
Wirthenson G, Vandewalle A, Guder WG (1981) Renal glycerol metabolism and the distribution of glycerol kinase in rabbit nephron. Biochem J 198: 543–549
Zablocki K, Bryla J (1988) Effect of glycerol on gluconeogenesis in isolated rabbit kidney-cortex tubules. Biochim Biophys Acta 970: 231–240
Zablocki K, Bryla J (1989)Utilization of alanine for glucose formation in isolated rabbit kidney-cortex tubules. FEBS Lett 259: 144–148
Zablocki K, Gemel J, Bryla J (1983) The inhibitory effect of octanoate, palmitate and oleate on glucose formation in rabbit kidney tubules. Biochim Biophys Acta 757: 111–118
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lietz, T., Rybka, J. & Bryla, J. Fatty acids and glycerol or lactate are required to induce gluconeogenesis from alanine in isolated rabbit renal cortical tubules. Amino Acids 16, 41–58 (1999). https://doi.org/10.1007/BF01318884
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01318884