Abstract
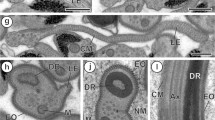
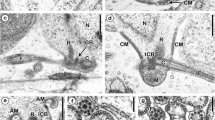
Pronounced sperm dimorphism is reported for the first time in the prosobranch order Vetigastropoda. Using transmission electron microscopy, it is demonstrated thatZalipais laseroni Kershaw (Trochoidea: Skeneidae) produces uniflagellate euspermatozoa (eupyrene, fertile sperm) and multiflagellate paraspermatozoa (oligopyrene, infertile sperm). Euspermatozoa show the following features: (1) a conical acrosomal vesicle; (2) a long tubular, helically coiled nucleus; (3) a short midpiece (mitochondrial sleeve surrounding a 3µm-long electron-dense rod); (4) a chambered body (? fused centrioles) continuous with the dense rod of the midpiece; (5) a flagellum (characterized by an electron-dense sheath surrounding and partly obscuring the central pair of tubules). Paraspermatozoa are composed of an elongate head (lacking an acrosomal complex), a short midpiece (centriolar rods interspersed with mitochondria), and a posterior tuft of flagella. The head consists of a rodshaped anterior body and a condensed nuclear remnant — the latter lodged in a shallow invagination of the anterior body. Multiple flagella are attached via centriolar rods to a layer of dense material lining the nuclear remnant membrane. During paraspermatozoan development, the nucleus partially degenerates, then condenses, while the endoplasmic reticulum (ER), apparently assisted by the Golgi complex, is responsible for production of numerous, electron-dense secretory vesicles. These vesicles subsequently fuse to form the elongate, anterior body of the head region. The ability of at least one line of trochoid gastropods to produce an oligopyrene, multiaxonemal paraspermatozoon, suggests that the Caenogastropoda (with this feature) might have been derived from the Vetigastropoda rather than from any other archaeogastropod source.
Similar content being viewed by others
Literature cited
Azevedo, C., Lobo-Da-Cunha, A., Oliveira, E. (1985). Ultrastructure of the spermatozoon inGibbula umbilicalis (Gastropoda, Prosobranchia), with special reference to acrosomal formation. J. submicrosc. Cytol. 17: 609–614
Azevedo, C., Oliveria, E. (1984). Two types of spermatozoa inGibbula umbilicalis. Cienc. biol. (Ser. C) 9: 25–26
Cox, L. R. (1960). Thoughts on the classification of the Gastropoda. Proc. malac. Soc. Lond. 33: 239–261
Daddow, L. Y. M. (1983). A double lead stain method for enhancing contrast of ultrathin sections in electron microscopy: a modified multiple staining technique. J. Microscopy 129: 147–153
Franzén, A. (1955). Comparative morphological investigations into the spermiogenesis among Mollusca. Zool. Bidr. Upps. 30: 399–456
Franzén, A. (1956). On spermiogenesis, morphology of the spermatozoon and biology of fertilization among invertebrates. Zool. Bidr. Upps. 31: 356–482
Gall, J. G. (1961). Centriole replication. A study of spermatogenesis in the snailViviparus. J. biophys. biochem. Cytol. 10: 163–193
Garreau de Loubresse, N. (1971). Spermiogenèse d'un gastéropode prosobranche:Nerita senegalensis: evolution du canal intranucleaire. J. Microscopie 12: 425–440
Giusti, F., Selmi, M. G. (1982a). The morphological peculiarities of the typical spermatozoa ofTheodoxus fluviatilis (L.) (Neritoidea) and their implications for motility. J. Ultrastruct. Res. 78: 166–177
Giusti, F., Selmi, M. G. (1982b). The atypical sperm in the prosobranch molluscs. Malacologia 22: 171–181
Griffond, B. (1980). Etude ultrastructurale de la spermatogenèse typique deViviparus viviparus (L.), mollusque gastéropode. Archs Biol., Bruxelles 91: 445–462
Griffond, B. (1981). Etude ultrastructurale de la spermatogenèse atypique deViviparus viviparus (L.), mollusque gastéropode. Archs Biol., Bruxelles 92: 275–286
Hachiri, S., Higashi, S. (1972). Spermiogenesis in the melanian snailsSemisulcospira decipiens andSemisulcospira niponica. Mem. Fac. Educ. Shiga Univ. nat. Sciences 21: 43–51
Haszprunar, G. (1988). On the origin and evolution of major gastropod groups, with special reference to the Streptoneura. J. mollusc. Stud. 54: 367–441
Healy, J. M: (1982). Ultrastructure of paraspermatozoa, euspermatozoa and eusperm-like spermatozoa ofObtortio cf.fulva (Prosobranchia Cerithiacea). Helgoländer Meeresunters. 35: 489–500
Healy, J. M. (1983). Ultrastructure of euspermatozoa of cerithiacean gastropods (Prosobranchia: Mesogastropoda). J. Morph. 178: 57–75
Healy, J. M. (1984). The ultrastructure of gastropod spermatozoa and spermiogenesis. Ph. D. thesis. University of Queensland
Healy, J. M. (1986a). Ultrastructure of paraspermatozoa of cerithiacean gastropods (Prosobranchia: Mesogastropoda). Helgoländer Meeresunters. 40: 177–199
Healy, J. M. (1986b). Euspermatozoa and paraspermatozoa of the relict cerithiacean gastropod,Campanile symbolicum (Prosobranchia, Mesogastropoda). Helgoländer Meeresunters. 40: 201–218
Healy, J. M. (1987). Spermatozoan ultrastructure and its bearing on gastropod classification and evolution. Aust. Zool. 24: 108–113
Healy, J. M. (1988). Sperm morphology and its systematic importance in the Gastropoda. Malac. Rev. (Suppl.) 4: 251–266
Healy, J. M. (1989). Ultrastructure of spermiogenesis in the gastropodCalliotropis glyptus Watson (Prosobranchia: Trochidae), with special reference to the embedded acrosome. Gamete Res. 24: 9–20
Healy, J. M., Jamieson, B. G. M. (1981). An ultrastructural examination of developing and mature paraspermatozoa inPyrazus ebeninus (Mollusca, Gastropoda, Potamididae). Zoomorphology 98: 101–119
Houbrick, R. S. (1981). Anatomy, biology and systematics ofCampanile symbolicum with reference to adaptive radiation in the Cerithiacea. Malacologia 21: 263–289
Ishizaki, T., Kato, K. (1958). The fine structure of atypical spermatozoa of the pond snailViviparus malleatus. Zool. Mag., Tokyo 67: 286–295
Kohnert, R., Storch, V. (1983). Ultrastrukturelle Untersuchungen zur Morphologie und Genese der Spermien von Archaeogastropoda. Helgoländer Meeresunters. 36: 77–84
Kohnert, R., Storch, V. (1984a). Vergleichend-ultrastrukturelle Untersuchungen zur Morphologie eupyrener Spermien der Monotocardia (Prosobranchia). Zool. Jb. 111: 51–93
Kohnert, R., Storch, V. (1984b). Elektronenmikroskopische Untersuchungen zur Spermiogenese der eupyrenen Spermien der Monotocardia (Prosobranchia). Zool. Jb. 112: 1–32
Koike, K. (1985). Comparative ultrastructural studies on the spermatozoa of the Prosobranchia (Mollusca: Gastropoda). Sci. Rep. Fac. Educ., Gunma Univ. 34: 33–153
Melone, G., Lora Lamia Donin, D., Cotelli, F. (1980). The paraspermatic cell (atypical spermatozoon) of the Prosobranchia: a comparative ultrastructural study. Acta zool., Stockh. 61: 191–201
Nishiwaki, S. (1964). Phylogenetical study on the type of the dimorphic spermatozoa in Prosobranchia. Sci. Rep. Tokyo Kyoiku Daig. (Sect. B) 11: 237–275
Rouse, G. W., Jamieson, B. G. M. (1987). An ultrastructural study of the spermatozoa of the polychaetesEurythoe complanata (Amphinomidae),Clymenella laseroni andMicromaldane laseroni (Maldanidae) with definition of sperm types in relation to reproductive biology. J. submicrosc. Cytol. 19: 573–584
Salvini-Plawen, L., Haszprunar, G. (1987). The Vetigastropoda and the systematics of streptoneurous Gastropoda (Mollusca). J. Zool., Lond. 211: 747–770
Selmi, M. G., Giusti, F. (1980). Structure and function in typical and atypical spermatozoa of Prosobranchia (Mollusca). 1.Cochlostoma montanum (Issel) (Mesogastropoda). Atti Accad. Fisiocr. Siena 1980: 115–167
Yasuzumi, G., Tanaka, H. (1958). Spermatogenesis in animals as revealed by electron microscopy. VI. Researches on the spermatozoon dimorphism in a pond snailCipangopaludina malleata. J. biophys. biochem. Cytol. 4: 621–632
Author information
Authors and Affiliations
Additional information
Communicated by G. F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Healy, J.M. Euspermatozoa and paraspermatozoa in the trochoid gastropodZalipais laseroni (Trochoidea: Skeneidae). Mar. Biol. 105, 497–507 (1990). https://doi.org/10.1007/BF01316321
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01316321