Abstract
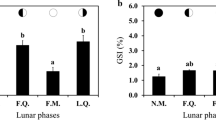
Gonad and blood samples were taken from the captive female Japanese sardineSardinops melanostictus between 1988 and 1989, and changes in serum levels of gonadal steroids were correlated with the annual gonadal cycle. Under captive conditions, female fish did not mature and spawn spontaneously, although oocytes developed up to the end of vitellogenic growth. Based on evidence from ovarian histology, the annual gonadal cycle of the Japanese sardine was divisible into four periods, i.e., immature (June to October), vitellogenesis (November to December), spawning (January to March), and post-spawning (April to May). The pattern of seasonal change in the gonadosomatic index (GSI) showed an inverse correlation to change in water temperature and reflected the degree of ovarian maturity. The serum estradiol-17β level increased from its lowest concentration (0.12 ng ml−1) in September to a peak (1.14 ng ml−1) in March. Serum 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-P) was detectable at low levels (<0.3 ng ml−1) between October and February, but was below the assay detection limit (0.06 ng ml−1) at all other times. Testosterone was not detectable (<0.06 ng ml−1) in the serum of any fish throughout the year. The effects of several steroids on the maturation of follicle-enclosed oocytes of sardine were examined in vitro, and 17α,20β-P was found to be the most potent inducer of maturation. This suggests that post-vitellogenic oocytes of the Japanese sardine in captivity have an ability to respond to an appropriate hormonal effector and subsequently to resume meiotic maturation.
Similar content being viewed by others
Literature cited
Asami, T. (1953). Studies on the ovarian eggs of clupeoid fishes. Bull. Jap. Soc. scient. Fish. 19: 398–404 (in Japanese with English summary)
Burke, M. G., Leatherland, J. F., Sumpter, J. P. (1984). Seasonal changes in serum testosterone, 11-ketotestosterone, and 17β-estradiol levels in the brown bullhead,Ictalurus nebulosus Lesueur. Can. J. Zool. 62: 1195–1199
Campbell, C. M.., Walsh, J. M., Idler, D. R. (1976). Steroids in the plasma of the winter flounder (Pseudopleuronectes americanus Walbaum): a seasonal study and investigation of steroid involvement in oocyte maturation. Gen. comp. Endocr. 29: 14–20
Crim, L. W., Idler, D. R. (1978). Plasma gonadotrophin, estradiol, and vitellogenin and gonad phosvitin levels in relation to the seasonal reproductive cycles of female brown trout. Annls Biol. anim. Biochim. Biophys. 18: 1001–1005
De Vlaming, V., Fitzgerald, R., Delahunty, G., Cech, J. J., Selman K., Barkley, M. (1984). Dynamics of oocyte development and related changes in serum estradiol 17-β, yolk precursor and lipid levels in the teleostean fishLeptocottus armatus. Comp. Biochem. Physiol. 77A: 599–610
Dindo, J. J., MacGregor, R. (1981). Annual cycle of serum gonadal steroids and serum lipids in striped mullet. Trans. Am. Fish. Soc. 110: 403–409
Donaldson, E. M., Hunter, G. A. (1983). Induced final maturation, ovulation, and spermiation in cultured fish. In: Hoar, W. S., Randall, D. J., Donaldson, E. M. (eds.) Fish physiology, Vol. IXB. Academic Press, New York, p. 351–403
Dye, H. M., Sumpter, J. P., Fagerlund, U. H. M., Donaldson, E. M. (1986). Changes in reproductive parameters during the spawning migration of pink salmon,Oncorhynchus gorbuscha (Walbaum). J. Fish Biol. 29: 167–176
Fitzpatrich, M. S., Van Der Kraak, G., Schreck, C. B. (1986). Profiles of plasma sex steroids and gonadotropin in coho salmon,Oncorhynchus kisutch, during final maturation. Gen. comp. Endocr. 62: 437–451
Goetz, F. W. (1983). Hormonal control of oocyte final maturation and ovulation in fishes. In: Hoar, W. S., Randall, D. J., Donaldson, E. M. (eds.) Fish physiology, Vol. IXB. Academic Press, New York, p. 117–170
Goetz, F. W., Bergman, H. L. (1978). The effects of steroids on final maturation and ovulation of oocytes from brook trout (Salvelinus fontinalis) and yellow perch (Perca flavescens). Biol. Reprod. 18: 293–298
Hiramoto, K. (1973). Studies on the life of the Japanese sardine,Sardinops melanosticta (Temminck et Schlegel), off the Boso Peninsula and in Tokyo Bay, central Japan — I. Aggregation of immatures and adults, and maturation of I-aged fish. Jap. J. Ecol. 23: 110–125 (in Japanese with English summary)
Hirose, K., Adachi, S., Nagahama, Y. (1985). Changes in plasma steroid hormone levels during sexual maturation in the ayuPlecoglossus altivelis. Bull. Jap. Soc. scient. Fish. 51: 398–403
Ishida, R., Ukawa, M., Arita, S. (1959). On the number of spawning times of sardineSardinops melanosticta (Temminck and Schlegel). Bull. Hokkaido reg. Fish. Res. Lab. 20: 139–146 (in Japanese with English summary)
Ito, S. (1961). Fishery biology of the sardine,Sardinops melanosticta (T. & S), in the waters around Japan. Bull. Japan Sea reg. Fish. Res. Lab. 9: 1–227 (in Japanese)
Kagawa, H., Takano, K., Nagahama, Y. (1981). Correlation of plasma estradiol-17β and progesterone levels with ultrastructure and histochemistry of ovarian follicles in the white-spotted char,Salvelinus leucomaenis. Cell Tissue Res. 218: 315–329
Kagawa, H., Young, G., Adachi, S., Nagahama, Y. (1982). Estradiol-17β production in amago salmon (Oncorhynchus rhodurus) ovarian follicles: role of the thecal layer and granulosa cells. Gen. comp. Endocr. 47: 440–448
Kagawa, H., Young, G., Nagahama, Y. (1983a). Relationships between seasonal plasma estradiol-17β and testosterone levels in vitro production by ovarian follicles of amago salmon (Oncorhynchus rhodurus). Biol. Reprod. 29: 301–309
Kagawa, H., Young, G., Nagahama, Y. (1983b). Changes in plasma steroid hormone levels during gonadal maturation in female goldfishCarassius auratus. Bull. Jap. Soc. scient. Fish. 49: 1783–1789
Kagawa, H., Young, G., Nagahama, Y. (1984). In vitro estradiol-17β and testosterone production by ovarian follicles of the goldfish,Carassius auratus. Gen. comp. Endocr. 54: 139–143
Kobayashi, M., Aida, K., Hanyu, I. (1986). Annual changes in plasma levels of gonadotropin and steroid hormones in goldfish. Bull. Jap. Soc. scient. Fish. 52: 1153–1158
Lam, T. J. (1983). Environmental influences on gonadal activity in fish. In: Hoar, W. S., Randall, D. J., Donaldson, E. M. (eds.) Fish physiology, Vol. IX B. Academic Press, New York, p. 65–116
Lamba, V., Goswami, S. V., Sundararaj, B. I. (1983). Circannual and circadian variations in plasma levels of steroids (cortisol, estradiol-17β, estrone, and testosterone) correlated with the annual gonadal cycle in the catfish,Heteropneustes fossilis (Bloch). Gen. comp. Endocr. 50: 205–225
Lambert, J. D. G., Bosman, G. I. C. G. M., Van den Hurk, R., Van Oordt, P. G. W. J. (1978). Annual cycle of plasma oestradiol-17β in the female troutSalmo gairdneri. Annls Biol. anim. Biochim. Biophys. 18: 923–927
MacGregor, R., Dindo, J. J., Finucane J. H. (1981). Changes in serum androgens and estrogens during spawning in bluefish,Pomatomus saltator, and king mackerel,Scomberomorus cavalla. Can. J. Zool. 59: 1749–1754
Matsuyama, M., Adachi, S., Nagahama, Y., Maruyama, K., Matsuura, S. (1990). Diurnal rhythm of serum steroid hormone levels in the Japanese whiting,Sillago japonica, a daily-spawning teleost. Fish Physiol. Biochem. 8: 329–338
Matsuyama, M., Adachi, S., Nagahama, Y., Matsuura, S. (1988). Diurnal rhythm of oocyte development and plasma steroid hormone levels in the red sea bream,Pagrus major, during the spawning season. Aquaculture, Amsterdam 73: 357–372
Nagahama, Y. (1987). Gonadotropin action on gametogenesis and steroidogenesis in teleost gonads. Zool. Sci. 4: 209–222
Nagahama, Y., Adachi, S. (1985). Identification of maturation-inducing steroid in a teleost, the amago salmon (Oncorhynchus rhodurus). Devl Biol. 109: 428–435
Nakai, Z. (1962). Studies of influences of environmental factors upon fertilization and development of the Japanese sardine eggs — with some reference to the number of their ova. Bull. Tokai reg. Fish. Res. Lab. 9: 109–150
Nakai, Z., Usami, S. (1962). Seasonal fluctuation in the sexual maturity of the Japanese sardine. Bull. Tokai reg. Fish. Res. Lab. 9: 151–171 (in Japanese with English summary)
Ouchi, K., Adachi, S., Nagahama, Y. (1988). Changes in plasma levels of steroid hormones during sexual maturation of female red seabreamPagrus major. Nippon Suisan Gakk. 54: 585–591 (in Japanese with English summary)
Ouchi, K., Adachi, S., Nagahama, Y., Matsumoto, A. (1985). Ovarian maturity and plasma steroid hormone levels in the cultured and wild yellowtail (Seriola quinqueradiata) during spawning season. Bull. natn. Res. Inst. Aquaculture 7: 13–20 (in Japanese with English summary)
Pankhurst, N. W. (1985). Final maturation and ovulation of oocytes of the goldeye,Hiodon alosoides (Rafinesque), in vitro. Can. J. Zool. 63: 1003–1009
Pankhurst, N. W., Conroy, A. M. (1987). Seasonal changes in reproductive condition and plasma levels of sex steroids in the blue cod,Parapercis colias (Bloch and Schneider) (Mugiloididae). Fish Physiol. Biochem. 4: 15–26
Pankhurst, N. W., Conroy, A. M. (1988). Endocrine changes during gonadal maturation and spawning in the orange roughy (Hoplostethus atlanticus Collett), a teleost from the midslope waters off New Zealand. Gen. comp. Endocr. 70: 262–273
Pankhurst, N. W., Stacey, N. E., Van Der Kraak, G. (1986). Reproductive development and plasma levels of reproductive hormones of goldeye,Hiodon alosoides (Rafinesque), taken from the North Saskatchewan River during the open-water season. Can. J. Zool. 64: 2843–2849
Rosenblum, P. M., Pudney, J., Callard, I. P. (1987). Gonadal morphology, enzyme histochemistry and plasma steroid levels during the annual reproductive cycle of male and female brown bullhead catfish,Ictalurus nebulosus Lesueur. J. Fish Biol. 31: 325–341
Santos, A. J. G., Furukawa, K., Kobayashi, M., Bando, K., Aida, K., Hanyu, I. (1986). Plasma gonadotropin and steroid hormone profiles during ovulation in the carpCyprinus carpio. Bull. Jap. Soc. scient. Fish. 52: 1159–1166
Scott, A. P., Bye, V. J., Baynes, S. M. (1980). Seasonal variations in sex steroids of female rainbow trout (Salmo gairdneri Richardson). J. Fish Biol. 17: 587–592
Scott, A. P., Mackenzie, D. S., Stacey, N. E. (1984). Endocrine changes during natural spawning in the white sucker,Catostomus commersoni. II. Steroid hormones. Gen. comp. Endocr. 56: 349–359
Scott, A. P., Sumpter, J. P. (1983). A comparison of the female reproductive cycles of autumn-spawning and winter-spawning strains of rainbow trout (Salmo gairdneri Richardson). Gen. comp. Endocr. 52: 79–85
Scott, A. P., Sumpter, J. P., Hardiman, P. A. (1983). Hormone changes during ovulation in the rainbow trout (Salmo gairdneri Richardson). Gen. comp. Endocr. 49: 128–134
Shimizu, A., Aida, K., Hanyu, I. (1985). Endocrine profiles during the short reproductive cycle of an autumn-spawning bitterling,Acheilognathus rhombea. Gen. comp. Endocr. 60: 361–371
Stacey, N. E., Peter, R. E., Cook, A. F., Truscott, B., Walsh, J. M., Idler, D. R. (1983). Changes in plasma concentrations of gonadotropin, 17β-estradiol, testosterone, and 17α-hydroxy-20β-dihydroprogesterone during spontaneous and brain lesion induced ovulation in goldfish. Can. J. Zool. 61: 2646–2652
Statistics and Informations Department, Ministry of Agriculture, Forestry and Fisheries (1990). Production of fisheries and water culture. In: The 63rd statistical yearbook of Ministry of Agriculture, Forestry and Fisheries, Japan, p. 262–263
Trant, J. M., Thomas, P. (1988). Structure-activity relationships of steroids in inducing germinal vesicle breakdown of Atlantic croaker oocytes in vitro. Gen. comp. Endocr. 71: 307–317
Trant, J. M., Thomas, P. (1989). Isolation of a novel maturation-inducing steroid produced in vitro by ovaries of Atlantic croaker. Gen. comp. Endocr. 75: 397–404
Trant, J. M., Thomas, P., Shackleton, C. H. L. (1986). Identification of 17α,20β,21-trihydroxy-4-pregnen-3-one as the major ovarian steroid produced by the teleostMicropogonias undulatus during final oocyte maturation. Steroids 47: 89–99
Truscott, B., Idler, D. R., So, Y. P., Walsh, J. M. (1986). Maturational steroids and gonadotropin in upstream migratory sockeye salmon. Gen. comp. Endocr. 62: 99–110
Ueda, H., Hiroi, O., Hara, A., Yamauchi, K., Nagahama, Y. (1984). Changes in serum concentrations of steroid hormones, thyroxine, and vitellogenin during spawning migration of the chum salmon,Oncorhynchus keta. Gen. comp. Endocr. 53: 203–211
Usami, S. (1964). Fecundity of the Japanese sardine,Sardinops melanosticta (Temminck & Schlegel) — I. Process of maturation and the number of ova determined on the basis of ovum diameter measurement of the large-sized females taken from the Japan Sea. Bull. Tokai reg. Fish. Res. Lab. 38: 1–30 (in Japanese with English summary)
Usami, S. (1972). A note on sexual maturity of I-age sardine in the sea off Kanto District, Honshu, 1949 through 1951. Bull. Tokai reg. Fish. Res. Lab. 70: 25–36 (in Japanese with English summary)
Van Ree, G. E., Lok, D., Bosman, G. (1977). In vitro induction of nuclear breakdown in oocytes of the zebrafishBrachydanio rerio (Ham. Buch.). Effects of the composition of the medium and of protein and steroid hormones. Proc. K. Ned. Akad. Wet. (Sect. C) 80: 353–371
Whitehead, C., Bromage, N. R., Breton, B. (1983). Changes in serum levels of gonadotropin, oestradiol 17β and vitellogenin during the first and subsequent reproductive cycles of female rainbow trout. Aquaculture, Amsterdam 34: 317–326
Wingfield, J. C., Grimm, A. S. (1977). Seasonal changes in plasma cortisol, testosterone and oestradiol-17β in the plaice,Pleuronectes platessa L. Gen. comp. Endocr. 31: 1–11
Yamauchi, K., Kagawa, H., Ban, M., Kasahara, N., Nagahama, Y. (1984). Changes in plasma estradiol-17β and 17α,20β-dihy-droxy-4-pregnen-3-one levels during final oocyte maturation of the masu salmonOncorhynchus masou. Bull. Jap. Soc. scient. Fish. 50: 2137
Young, G., Crim, L. W., Kagawa, H., Kambegawa, A., Nagahama, Y. (1983). Plasma 17α,20β-dihydroxy-4-pregnen-3-one levels during sexual maturation of amago salmon (Oncorhynchus rhodurus): correlation with plasma gonadotropin and in vitro production by ovarian follicles. Gen. comp. Endocr. 51: 96–105
Young, G., Kagawa, H., Nagahama, Y. (1982). Secretion of aromatizableΔ 4 androgens by thecal layers during estradiol-17β production by ovarian follicles of amago salmon (Oncorhynchus rhodurus) in vitro. Biomed. Res. 3: 659–667
Zhu, Y., Furukawa, K., Aida, K., Hanyu, I. (1989). Annual reproductive rhythm of the tobinumeri-dragonetRepromucennus beniteguri (Callionymidae) in Lake Hamana. Nippon Suisan Gakk. 55: 591–599
Zohar, Y., Pagelson, G., Tosky, M. (1988). Daily changes in reproductive hormone levels in the female gilthead seabreamSparus aurata at the spawning period. In: Reproduction in fish — basic and applied aspects in endocrinology and genetics. Institut National de la Recherche Agronomique, Paris, p. 119–125
Author information
Authors and Affiliations
Additional information
Communicated by M. Anraku, Tokyo
Rights and permissions
About this article
Cite this article
Matsuyama, M., Adachi, S., Nagahama, Y. et al. Annual reproductive cycle of the captive female Japanese sardineSardinops melanostictus: Relationship to ovarian development and serum levels of gonadal steroid hormones. Mar. Biol. 108, 21–29 (1991). https://doi.org/10.1007/BF01313467
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01313467