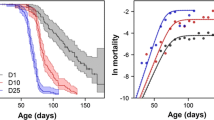
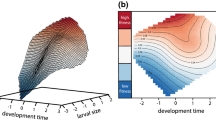
Abstract
The multivoltine, estuarine amphipodGammarus lawrencianus has four generations per year in an environment where temperatures range seasonally from −1° to 25°C. Temperature-response curves for rates of brood production and development were determined by laboratory experiments and field observation. The life history and population dynamics were observed over a full annual cycle (1981) for a field population located at Rocky Run, Porter's Lake, Nova Scotia, Canada. On a natural (i.e., sidereal) time scale, the generations appear to have very different life histories: the two summer generations have short lives, rapid development and mature at small size (classicr-selection), whereas the overwintering generations have relatively low rates of mortality, slow development and mature at large size (classicK-selection). This pattern (larger size at maturity at lower temperatures) is widespread in aquatic poikilotherms. Similar life-history differences are evident among cohorts of the summer generations that mature at different temperatures. When time is expressed on a physiological scale that removes the effect of temperature on embryonic development and reproductive rate, the variation within and among generations is greatly reduced. In particular, an apparent alternation betweenr- andK-selection largely disappears. Because the generations are temporally isolated, it might be surmised that natural selection acting on the summer generations might antagonize the effects of natural selection acting on the fall and winter generations. However, the scaling of the rates of development, maturation, growth, reproduction and mortality on the physiological time scale derived from the temperature dependence of development and reproductive rate gives a very different and more homogeneous pattern.
Similar content being viewed by others
Literature cited
Baker, R. J., Nelder, J. A. (1978). The GLIM system manual, Release 3. Oxford, University Press (Numerical Algorithms Group)
Berven, K. A., Gill, D. E. (1983). Countergradient selection in the green frog,Rana clamitans. Evolution, Lawrence, Kansas 33: 85–97
Birkhead, T. R., Clarkson, K. (1980). Mate selection and precopulatory guarding inGammarus pulex. Z. Tierpsychol. 52: 365–380
Brooks, J. L., Dodson, S. I. (1965). Predation, body size, and the composition of the plankton. Science, N.Y. 150: 28–35
Caine, E. A. (1979). Population structures of two species of caprellid amphipods (Crustacea). J. exp. mar. Biol. Ecol. 40: 115–135
Cooley, J. M. (1975). The effect of temperature on the development of the egg, first and second naupliar stages and vertical migration inDiaptomus oregonensis Lillj. (Copepod: Calanoidea). Master's thesis. University of Toronto
Doyle, R. W., Hunte, W. (1981). Genetic changes in the components of fitness of a crustacean population in a controlled environment. J. exp. mar. Biol. Ecol. 52: 147–156
Doyle, R. W., Myers, R. A. (1982). The measurement of the direct and indirect intensities of natural selection. In: Dingle, H., Hegmann, J. P. (eds.) Evolution and genetics of life histories. Springer-Verlag, New York, p. 177–186
Hartnoll, R. G. (1984). Growth. In: Abele, L. G. (ed.) The biology of the Crustacea. Vol. 2. Academic Press, New York, p. 111–195
Hartnoll, R. G., Smith, S. M. (1978). Pair formation and the reproductive cycle inGammarus duebeni. J. nat. Hist. 12: 501–511
Kahn, M. F. (1965). The effect of constant and varying temperatures on the development ofAcantocyclops viridis (Jurine). Proc. R. Ir. Acad. 64: 117–130
Keen, R., Parker, D. L. (1977). Determining expected duration under conditions of alternating temperatures. J. theor. Biol. 81: 599–607
Laudien, H. (1973). Effect of temperature on the processes of growth and development. In: Precht, H., Christopherson, J., Hensel, H., Larcher, W. (eds.) Temperature and life. Springer Verlag, New York, p. 355–399
Logan, J. A., Wolkind, D. J., Hoyt, S. C., Tanigoshi, D. J. (1976). An analytical model for the description of temperature dependent rate phenomena in arthropods. Envir. Ent. 5: 1133–1140
McLaren, I. A., Corkett, C. J., Sillioux, E. J. (1966). Temperature adaptations of copepod eggs from the arctic to the tropics. Biol. Bull. mar. biol. Lab., Woods Hole 137: 486–493
Myers, R. A., Runge, J. (1983). Predictions of seasonal natural mortality rates in a copepod population using life history theory. Mar. Ecol. Prog. Ser. 11: 189–194
Nelson, W. G. (1979). Experimental studies of selective predation on amphipods: consequences for amphipod distribution and abundance. J. exp. mar. Biol. Ecol. 38: 225–245
Nelson, W. G. (1980). Reproduction patterns of gammaridean amphipods. Sarsia 65: 61–71
Nilsson, L. M. (1977). Incubation time, growth and mortality of the amphipodGammarus pulex under laboratory conditions. Oikos 29: 93–98
Pielou, E. C. (1977). Mathematical ecology. John Wiley & Sons, New York
Ricker, W. E. (1969). Effects of size selective mortality and sampling bias on estimates of growth, mortality, production and yield. J. Fish. Res. Bd Can. 26: 479–541
Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Bd Can. 191: 1–382
Robertson, A. I., Howard, R. K. (1978). Diel trophic interactions between vertically-migrating zooplankton and their fish predators in an eelgrass community. Mar. Biol. 48: 207–213
Smith-Gill, S. J. (1983). Developmental plasticity: developmental conversion versus phenotypic modulation. Am. Zool. 23: 47–58
Steele, D. H., Steele, V. J. (1969). The biology ofGammarus (Crustacea, Amphipoda) in the northwestern Atlantic, I.Gammarus duebeni Lillj. Can. J. Zool. 47: 235–244
Steele, D. H., Steele, V. J. (1970). The biology ofGammarus (Crustacea, Amphipoda) in the northwestern Atlantic. IV.Gammarus lawrencianus Bousfield. Can. J. Zool. 48: 1261–1267
Steele, D. H., Steele, V. J. (1975). The biology ofGammarus (Crustacea, Amphipoda) in the northwestern Atlantic. XI. Comparison and discussion. Can. J. Zool. 53: 1116–1126
Steele, D. H., Steele, V. J. (1986). The cost of reproduction in the amphipodGammarus lawrencianus. Crustaceana 51: 176–182
Steele, V. J. (1981). The effect of photoperiod on the reproductive cycle ofGammarus lawrencianus Bousfield. J. exp. mar. Biol. Ecol. 53: 1–7
Stinner, R. E., Gutierrez, A. P., Butler, G. D. (1974). An algorithm for temperature dependent growth rate simulation. Can. Ent. 106: 519–524
Sutcliffe, D. W., Carrick, T. R. (1981). Effect of temperature on the duration of egg development, molting and growth in juveniles ofCrangonyx pseudogracilis (Crustacea: Amphipoda) in the laboratory. Freshwat. Biol. 11: 511–522
Sutcliffe, D. W., Carrick, T. R., Willoughby, L. G. (1981). Effect of diet, body size, age and temperature on growth rates in the amphipodGammarus pulex. Freshwat. Biol. 11: 183–214
Taylor, B. J. R. (1965). The analysis of polymodal frequency distributions. J. Anim. Ecol. 34: 445–452
Taylor, F. (1979). Convergence to the stable age distribution in populations of insects. Am. Nat. 113: 511–530
Taylor, F. (1981). Ecology and evolution of physiological time in insects. Am. Nat. 117: 1–23
Threlkeld, S. (1979). Estimating cladoceran birth rates: the importance of egg mortality and the egg age distribution. Limnol. Oceanogr. 24: 601–612
Vannote, R. L. (1978). A geometric model describing quasi-equilibrium of energy flow in a population of stream insects. Proc. nat. Acad. Sci. U.S.A. 75: 381–384
Vince, S., Valiela, I., Backus, N., Teal, J. M. (1976). Predation by the salt marsh killifishFundulus heteroclitus (L.) in relation to prey size and habitat structure: consequences for prey distribution and abundance. J. exp. mar. Biol. Ecol. 23: 255–266
Author information
Authors and Affiliations
Additional information
Communicated by M. G. Hadfield, Honolulu
Rights and permissions
About this article
Cite this article
Sinervo, B., Doyle, R.W. Life-history analysis in “physiological” compared with “sidereal” time: An example with an amphipod (Gammarus lawrencianus) in a varying environment. Mar. Biol. 107, 129–139 (1990). https://doi.org/10.1007/BF01313250
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01313250