Objective
Our objective was to obtain parthenogenetic activation of unfertilized human oocytes by puromycin and to try to use this procedure for cytogenetic purposes.
Setting
The setting was our IVF laboratory.
Methods
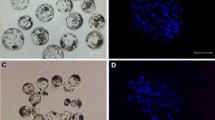
Eighty-two spare oocytes from 34 IVF patients were used. In the first series of experiments 39 unfertilized oocytes were cultured in medium containing 100, 50, or 10 µg/ml puromycin for 6 to 24 hr. After the appearance of pronuclei they were transferred to plain medium, further cultured, and cytogenetically analyzed. In the second series of experiments 43 oocytes were cultured for 5 to 10 hr in 10 µg/ml puromycin, transferred to plain medium, and fixed for cytogenetic analysis 2 hr after nuclear envelope breakdown.
Results
Ninety-one percent of the oocytes in the first experiment showed the presence of one or more nuclei. From the pronucleate oocytes additionally cultured in puromycin-free medium, 46% developed further to the metaphase of the first mitotic division or the two-cell stage and 54% remained arrested at the pronuclear stage. In the second experiment 88% of the treated oocytes showed pronuclei or had cleaved, and after with-drawal from puromycin 96% of the pronucleate oocytes entered mitosis.
Conclusion
Puromycin induces haploid as well as diploid parthenogenesis in aged human oocytes. A 5- to 10-hr treatment of oocytes with 10 µg/ml puromycin yields the highest percentage of activation, and almost all parthe-nogenetically activated oocytes enter or develop beyond the first cleavage mitosis. Analysis of mitotic metaphase chromosomes from parthenogenetically activated human oocytes may be a promising new approach to preimplantation cytogenetics.
Similar content being viewed by others
References
Graham CF: The production of parthenogenetic mammalian embryos and their use in biological research. Biol Rev 1974;49:399–422
Tarkowski AK: Induced parthenogenesis in the mouse.In 33rd Symposium of the Society of Developmental Biology: Developmental Biology of Reproduction, CL Markert (ed). New York, Academic Press, 1975, pp 107–129
Whittingham DG: Parthenogenesis in mammals.In Oxford Reviews of Reproductive Biology, CA Finn (ed). Oxford, Oxford University Press, 1980, Vol 2, pp 205–231
Kaufman MH: Early Mammalian Development: Parthenogenetic Studies. Cambridge and London, Cambridge University Press, 1983
Dyban AP, Noniashvili EM: Parthenogenesis in mammals. Ontogenez 1986;17:368–388
Dyban AP, Baranov VS: Cytogenetics of Mammalian Embryonic Development. Oxford, Oxford University Press, 1987
Muechler EK, Graham MC, Huang K, Partridge AB, Jones K: Parthenogenesis of human oocytes as a function of vacuum pressure. J Vitro Fert Embryo Transfer 1989;6:335–337
Pickering SJ, Johnson MH, Braude PR, Houliston E: Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod 1988;3:978–989
Johnson MH, Pickering SJ, Braude PR, Vincent C, Cant A, Currie J: Acid Tyrode's solution can stimulate parthenogenetic activation of human and mouse oocytes. Fertil Steril 1990;53:266–270
Winston N, Johnson M, Pickering S, Braude P: Parthenogenetic activation and development of fresh and aged human oocytes. Fertil Steril 1991;56:904–912
Siracusa G, Whittingham DG, Molinaro M, Vivarelli E: Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol 1978;43:157–166
Clarke HJ, Rossant J, Masui Y: Suppression of chromosome condensation during meiotic maturation induces parthenogenetic development of mouse oocytes. Development 1988;104:97–103
Whittingham DG: Culture of mouse ova. J Reprod Fertil 1971;Suppl 14:7–21
Quinn P, Barros C, Whittingham DG: Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil 1982;66:161–168
Dyban AP: An improved method for chromosome preparations from preimplantation mammalian embryos, oocytes or isolated blastomeres. Stain Technol 1983;58:69–72
Dyban AP: Reliable technique for chromosomal preparations from mammalian oocytes, preimplantation embryos and isolated blastomeres.In Preimplantation Genetics, Y Verlinsky, A Kuliev (eds). New York, Plenum Press, 1991, pp 293–298
Clarke HJ, Masui Y: The induction of reversible and irreversible chromosome decondensation by protein synthesis inhibition during meiotic maturation of mouse oocytes. Dev Biol 1983;97:291–301
Dyban AP, Khozhai LE: Parthenogenetic development of ovulated mouse ova induced by ethyl alcohol. Byull eksp Biol Med 1980;89:487–489
Cuthbertson KSR: Parthenogenetic activation of mouse oocytes in vitro with ethanol and benzyl alcohol. J Exp Zool 1982;226:311–314
Dyban AP, Noniashvili EM: The factors determining the incidence and the rate of parthenogenetic development in mouse eggs activated by ethyl alcohol treatment. Ontogenez 1985;16:582–597
Dyban AP, Noniashvili EM: Parthenogenetic development of mouse eggs activated by heat shock. Ontogenez 1986;17:587–598
Balakier H, Tarkowski A: Diploid parthenogenetic mouse embryos produced by heat shock and cytochalasin B. J Embryol Exp Morphol 1976;35:25–39
Komar A: Parthenogenetic development of mouse eggs activated by heat-shock. J Reprod Fert 1973;35:433–443
Plachot M, Mandelbaum J, Junca AM, de Grouchy J, Salat-Baroux J, Cohen J: Cytogenetic analysis and developmental capacity of normal and abnormal embryos after IVF. Hum Reprod 1989;4(Suppl):99–103
Plachot M: Chromosome analysis of oocytes and embryos.In Preimplantation Genetics, Y Verlinsky, A Kuliev (eds). New York, Plenum Press, 1991, pp 103–112
Surani MA, Kothary R, Allen ND, Singh PB, Fundele R, Ferguson-Smith AC, Barton SC: Genome imprinting and development in the mouse. Development 1990;Suppl;89–90
Schmiady H, Sperling K, Kentenich H, Stauber M: Prematurely condensed human sperm chromosomes after in vitro fertilization (IVF). Hum Genet 1986;74:441–443
Schmiady H, Kentenich H: Premature chromosome condensation after in-vitro fertilization. Hum Reprod 1989;4:689–695
De Sutter P: Investigation of Determining Factors for in Vitro Fertilization of Human Oocytes. Cytogenetic Analysis, Postdoctoral thesis. Ghent, University of Ghent, 1991, pp 72–130
Pieters MH, Geraedts JP, Dumoulin JC, Evers JL, Bras M, Kornips FH, Menheere PP: Cytogenetic analysis of in vitro fertilization (IVF) failures. Hum Genet 1989;81:367–370
Clarke HJ, Masui Y: Inhibition by dibutyryl cyclic AMP of the transition to metaphase of mouse oocyte nuclei and its reversal by cell fusion to metaphase oocytes. Dev Biol 1985;108:32–37
Boue JG, Boue A: Chromosome abnormalities and abortion.In Physiology and Genetics of Reproduction, Part B, EM Coutinho, F Fuchs (eds). New York, Plenum, 1974, pp 317–339
Boue JG, Boue A: Chromosome anomalies in early spontaneous abortion.In Current Topics in Pathology, A Gropp, K Benirschke (eds). Heidelberg, Springer, 1976, pp 193–208
Chandley AC: Infertility and chromosome abnormality. Oxford Rev Reprod Biol 1984;6:1–46
Epstein CJ: The Consequences of Chromosome Imbalance: Principles, Mechanisms and Models. New York, Cambridge University Press, 1987
De Sutter P, Dhont M, Vanluchene E, Vandekerckhove D: Correlations between follicular fluid steroid analysis and maturity and cytogenetic analysis of human oocytes that remained unfertilized after in vitro fertilization. Fertil Steril 1991;55:958–963
Chandley AC: Meiotic analysis in germ cells of man and the mouse.In Mammalian Development: A Practical Approach, M Monk (ed). Oxford and Washington, IRL Press, 1987, pp 71–91
De Sutter P, Dhont M, Verschraegen-Spae MR, Steyaert H, Corijn W, Leroy J, Vandekerckhove D: Chromosome analysis of human oocytes failing to fertilize in vitro: A mathematical model for the estimation of the first meiotic nondisjunction frequency. Hum Reprod 1991;6:550–554
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
De Sutter, P., Dozortsev, D., Cieslak, J. et al. Parthenogenetic activation of human oocytes by puromycin. J Assist Reprod Genet 9, 328–337 (1992). https://doi.org/10.1007/BF01203955
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01203955