Abstract
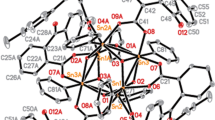
The crystal and molecular structure of the title complex has been determined. The space group isP21/n, witha=8.460(3),b=8.503(3),c=20.341 (8) Å,β=98.41(3) andZ=2. The ligand molecules chelate the CuII such that the metal has a tetragonally distorted octahedral geometry; four N atoms occupy the equatorial plane and two H2O molecules occupy the axial positions. The average Cu-N and Cu-O distances are 2.005(6) and 2.47(1) Å, respectively. The novel ligand, 1,1′-dimethyl-2,2′-diimidazolylsulfide was formedin situ from a methanolic solution of copper(II) sulfate pentahydrate and 1-methylimidazole-2-thiol. The measured spectral parameters (visible, EPR, and photoelectron spectroscopy) correlate well with the molecular structure.
Similar content being viewed by others
References
Agnus, Y., Louis, R., and Weiss, R. (1980)J. Chem. Soc. Chem. Commun., 867–869.
Baldwin, D. A., Copperthwaite, R. G., Faisca, A. M. M., and Markwell, A. J. (1982) unpublished results.
Birker, P. J. M. W. L., and Freeman, H. C. (1976)J. Chem. Soc. Chem. Commun., 312–313.
Birker, P. J. M. W. L., and Freeman, H. C. (1977)J. Am. Chem. Soc. 99, 6890–6899.
Birker, P. J. M. W. L., Reedijk, J., Verschoor, G. C., and Jordanov, J. (1982)Acta Cryst. B 38, 2245–2247.
Brundle, C. R., and Roberts, M. W. (1972)Proc. R. Soc. London A 331, 383.
De Rooij, J., Mijlhoff, F. C., and Renes, G. (1975)J. Mol. Struct. 25, 169.
Frost, D. C., Ishitani, A., and McDowell, C. A. (1972)Mol. Phys. 24, 861.
Goodman, B. A., and Raynor, J. B. (1970) InAdvances in Inorganic and Radiochemistry, Eméleus, H. J., and Sharpe, A. G., eds. (Academic Press, New York), pp. 313–324.
Jandal, P., Seip, H. M., and Torgrimsen, T. (1976)J. Mol. Struct. 32, 369.
Lever, A. B. P. (1968)Inorganic Electronic Spectroscopy (Elsevier, Amsterdam), pp. 355–360.
McFadden, D. C., McPhail, A. T., Garner, C. D., and Mabbs, F. E. (1976)J. Chem. Soc. Dalton Trans., 47–52.
McMillin, D. R., Rosenberg, R. C., and Gray, H. B. (1974)Proc. Natl. Acad. Sci. USA,71, 4760.
Nowell, I. W., Cox, A. G., and Raper, E. S. (1979).Acta Cryst. B 35, 3047–3050.
O'Neill, M. E., Raper, E. S., and Nowell, I. W., and Daniels, J. A. (1981)Inorg. Chim Acta 54, L243-L247.
Raper, E. S., and Nowell, I. W. (1979)Acta Cryst. B 35, 1600–1603.
Sakurai, H., Yokoyama, A., and Tanaka, H. (1970)Chem. Pharm Bull. 18, 2373–2385.
Sheldrick, G. M. (1976)Shelx 76. Program from the University of Cambridge, Cambridge, England.
Siegbahn, K. (1967)ESCA. Atomic, Molecular and Solid-State Structure Studied by Means of Electron Spectroscopy (Almqvist and Wiksells, Uppsala).
Solomon, E. I., Hare, J. W., and Gray, H. B. (1976)Proc. Natl. Sci. USA 73, 1389–1393.
Solomon, E. I., Hare, J. W., Dooley, D. M., Dawson, J. H., Stephens, P. J., and Gray, H. B. (1980)J. Am. Chem. Soc. 102, 168–177.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baldwin, D.A., Boeyens, J.C.A., Copperthwaite, R.G. et al. Crystal and molecular structure of diaquobis(1,1′-dimethyl-2,2′-diimidazolylsulfide)copper(II) methylsulfate, (C8H10N4S)2Cu(II)(OH2)2(CH3OS03)2 . Journal of Crystallographic and Spectroscopic Research 14, 157–167 (1984). https://doi.org/10.1007/BF01189557
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01189557