Abstract
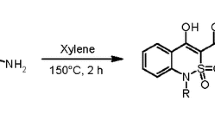
The course of the Vilsmeier acetylation of new heterocyclic systems, namely, indolo[4,5-d]-, indolo[6,5-d]-, indolo[5,6-d]-, indolo[5,4-d]benzo[b]furans, 3H-pyrrolo[2,3-c]carbazole, 3H-pyrrolo[2,3-c]pheno-thiazine 11,11-dioxide, and 3H-pyrrolo[2,3-c]acridine depends on the type of fusion of the pyrrole ring. Angular heterocycles are acetylated at the β-position of the pyrrole ring, while linear heterocycles under analogues conditions give dimerization products with a substituent at the nitrogen atom of the hydrogenated part of the dimer molecule. 3H-Pyrrolo[2,3-c]phenothiazine 11,11-dioxide and 3H-pyrrolo[2,3-c]acridine are not acetylated under Vilsmeier reaction conditions.
Similar content being viewed by others
References
T. E. Khostariya, M. L. Kakhabrishvili, M. I. Sikharulidze, L. N. Kurkovskaya, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 3, 355 (1985).
T. E. Khostariya, M. I. Sikharulidze, M. L. Kakhabrishvili, L. N. Kurkovskaya, and N. N. Suvorov, Soobshch. Akad. Nauk GruzSSR,117, No. 2, 333 (1985).
M. I. Sikharulidze, T. E. Khostariya, L. N. Kurkovskaya, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 8, 1087 (1979).
M. I. Sikharulidze, T. E. Khostariya, L. N. Kurkovskaya, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 4, 497 (1981).
T. E. Khostariya, G. A. Palavandishvili, M. I. Sikharulidze, L. N. Kurkovskaya, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 10, 1335 (1984).
T. E. Alyab'eva, T. E. Khostariya, L. G. Vasil'ev, L. G. Tret'yakova, T. K. Efimova, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 8, 1092 (1979).
T. E. Khostariya, M. L. Kakhabrishvili, L. N. Kurkovskaya, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 10, 1366 (1984).
T. E. Khostariya, M. I. Sikharulidze, L. G. Tret'yakova, T. K. Efimova, and N. N. Suvorov, Khim. Geterotsikl. Soedin., No. 6, 790 (1979).
G. A. Palavandishvili, T. E. Khostariya, L. N. Kurkovskaya, and N. N. Suvorov, KHim. Geterotsikl. Soedin., No. 12, 1637 (1981).
N. N. Suvorov, T. M. Alyab'eva, and T. E. Khostariya, Khim. Geterotsikl. Soedin., No. 9, 1277 (1978).
R. S. Sagitullin, A. N. Kost, E. D. Matveeva, and N. I. Nemudreva, Khim. Geterotsikl. Soedin., No. 7, 920 (1970).
L. B. Shagalov, T. A. Tkachenko, V. N. Eraksina, and N. N. Suvorov, Chemistry and Technology of Organic Compounds and High-Molecular-Weight Compounds, Proceedings of the D. I. Mendeleev Moscow Chemical Engineering Institute [in Russian], No. 8, Moscow (1974), p. 65.
Additional information
Georgian Technical University, 380075 Tbilisi, Georgia. D. E. Mendeleev Russian Chemical Engineering University, 125047 Moscow. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 10, pp. 1331–1336, October, 1996. Original article submitted August 19, 1996.
Rights and permissions
About this article
Cite this article
Khoshtariya, T.E., Kurkovskaya, L.N. & Suvorov, N.N. Acetylation of indolobenzo[b]furans, pyrrolocarbazole, pyrrolophenothiazine dioxide and pyrroloacridine under Vilsmeier reaction conditions. Chem Heterocycl Compd 32, 1141–1146 (1996). https://doi.org/10.1007/BF01169223
Issue Date:
DOI: https://doi.org/10.1007/BF01169223