Abstract
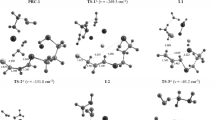
Models of the major end products and intermediate products, pre viously detected in a study of the mechanism of the interaction of trichloromethylarenes with pyridines, were calculated by a semiem pirical quantum chemical MNDO method. Models of some putative unstable intermediates of the key redox step of the process under consideration — the aromatization of 4-chloro- or 4-pyridino-substituted 1-(α,α-dichloroarylmethyl)-1,4-dihydropyridines with transfer of hydrogen from the 4-position of the dihydropyridine ring to the benzyl dichloromethylene group and the formation of N-(α-chloroarylmethyl)-4-cliloropyridiniuni chlorides of N-(α-chloroarylmethyl)-4-pyridinopyridinium dichlorides, were also calculated.
Similar content being viewed by others
References
D. B. Brokhovetskii, L. I. Belen'kii, and M. M Krayushkin, Izv. Akad. Nauk SSSR, Ser. Khim., No. 3, 748 (1989).
L. I. Belen'kii, D. B. Brokhovetskii, and M. M. Krayushkin, Tetrahedron,47, 447 (1991)
L. I, Belen'kii, I. S. Poddubnyi, and M. M. Krayushkin, Izv. Akad. Nauk, Ser. Khim., No. 11, 1928 (1993).
L. I. Belen'kii, I. S. Poddubnyi, and M. M. Krayushkin, Mendeleev Commun., No. 3, 97 (1993).
L. I. Belen'kii, I. S. Poddubnyi, and M. M. Krayushkin, Tetrah. Lett.,28, 5075 (1995).
L. I. Belen'kii, I. S. Poddubnyi, and M. M. Krayushkin, Khim. Geterotsikl. Soedin., No, 6, 830 (1995).
T. Clark, Computer Chemistry [Russian translation], Mir, Moscow (1990), p. 383.
B. Almarzoqi, A. V. George, and N. S. Isaacs, Tetrahedron,42, 601 (1986).
R. A. Olofson, D. M. Zimmerman, and R. C. Schnur, J. Labeled Compds.,8, 397 (1972).
E. Anders, F. Markus, H. Meske, J. G. Tropsch, and G. Maas, Chem. Ber., No. 120, 735 (1987),
E. Anders, J. G. Tropsch, A. R. Katritzky, D. Rasala, and J. J. Van den Eynde, J. Org. Chem.,54, 4808 (1989).
I. S. Poddubnyi, Khim. Geterotsiki. Soedin., No. 6, 774 (1995).
E. Koenigs and H. Greiner, Ber,, No. 64, 1049 (1931).
F. Krohnke, Angew Chem., No. 65, 605 (1953).
D. Jerchel, H, Fischer, and K. Thomas, Chem. Ber., No. 89, 2921 (1956).
K. Thomas and D. Jerchel, Angew. Chem., No. 70, 719 (1958).
A. K. Sheinkman, Khim. Geterotsikl. Soedin., No. l, 3 (1974).
J. P. Wibaut and F. W. Broekman, Rec. Trav. Chim.,80, 309 (1961).
A. L. Lehninger, D. L. Nelson, and M. M. Cox, Principles of Biochemistry, 2nd Ed., Worth Publishers, New York (1993), p. 554,
Ya. Stradyn, Ya. Ogle, and G. Duburs, Khim. Geterotsikl. Soedin., No. 1, 135 (1994).
R. G. Pearson, J. Amer. Chem. Soc.,110, 2092 (1988).
Additional information
N. D. Zelinksii Institute of Organic Chemistry, Russian Academy of Sciences, Moscow 117913. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 11, pp. 1551–1558, November, 1995. Original article submitted November 13, 1995
Rights and permissions
About this article
Cite this article
Belen'kii, L.I., Chuvylkin, N.D. Quantum chemical investigation of the mechanism of the reaction of pyridines with trichloromethylarenes. Chem Heterocycl Compd 31, 1346–1352 (1995). https://doi.org/10.1007/BF01168630
Issue Date:
DOI: https://doi.org/10.1007/BF01168630