Abstract
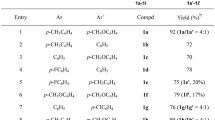
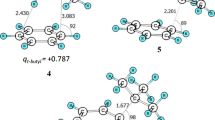
We consider a radical-ion mechanism for dehydroaromatization of benzimidazolines by triarylmethyl cations, including as the initial step electron transfer from the hydroheteroaromatic compound to the electrophilic reagent. We draw the conclusion that there is significant weakening of the C(2)-H bond in the benzimidazoline radical cation formed in this stage.
Similar content being viewed by others
References
A. V. El'tsov and M. Z. Girshovich, Zh. Org. Khim.,3, 1332 (1967).
A. V. El'tsov, N. V. Pavlish, and V. A. Ketlinskii, Zh. Org. Khim.,14, 1751 (1978).
A. S. Morkovnik, Author's Abstract, Dissertation in competition for the academic degree of Doctor of the Chemical Sciences, Rostov-na-Donu (1991).
O. Yu. Okhlobystin and N. T. Berberova, Khim. Geterotsikl. Soedin., No. 8, 1011 (1984).
N. T. Berberova, E. P. Ivakhnenko, A. S. Morkovnik, and O. Yu. Okhlobystin, Khim. Geterotsikl. Soedin., No. 12, 1696 (1979).
A. S. Morkovnik, E. S. Klimov, A. N. Suslov, E. P. Ivakhnenko, O. Yu, Okhlobystin, and B. A. Tertov, Khim. Geterotsikl. Soedin., No. 5, 634 (1987).
A. S. Morkovnik, A. N. Suslov, and B. A. Tertov, Zh. Obhsh. Khim.,61, 471 (1991).
M. M. Baizer (ed.), Organic Electrochemistry [Russian translation], Mir, Moscow (1976).
A. S. Morkovnik, E. P. Ivakhnenko, Yu. G. Bogachev, B. A. Tertov, N. T. Berberova, and O. Yu. Okhlobystin, Khim. Geterotsikl. Soedin., No. 2, 203 (1988).
H. Volz and W. Lotsch, Tetrahed. Lett., No. 27, 2275 (1969).
L. Eberson and F. Radner, Acta Chem. Scand.,38B, 861 (1984).
L. Eberson, Electron Transfer Reactions in Organic Chemistry, Springer Verlag, Berlin (1987), p. 32.
A. N. Suslov, A. S. Morkovnik, and G. S. Borodkin, Teor. Éksp. Khim., No. 6, 658 (1990).
L. Eberson, Adv. Phys. Org. Chem.,18, 79 (1982).
V. A. Rodionov and É. G. Rozantsev, Long-Lived Radicals [in Russian], Nauka, Moscow (1972), p. 22.
A. A. Khapicheva, N. T. Berberova, E. S. Klimov, and O. Yu. Okhlobystin, in: Abstracts, Sixth All-Union Conference on Problems of Charge-Transfer Complexes and Radical-Ion Salts [in Russian], Chernogolovks (1984), p. 180.
V. E. Titov and V. G. Koshechko, in: Abstracts, Sixth All-Union Conference on Problems of Charge-Transfer Complexes and Radical-Ion Salts [in Russian], Chernogolovka (1984), p. 178.
A. A. Khapicheva, N. T. Berberova, E. S. Klimov, and O. Yu. Okhlobystin, Zh. Obshch. Khim.,55, 1533 (1985).
R. W. Alder, M. Bonifacic, and K.-D. Asmus, J. Chem. Soc., Perkin Trans. 11, No. 2, 277 (1986).
A. I. Shif, Dissertation in competition for the academic degree of Candidate of the Chemical Sciences, Rostov-na-Donu (1989).
Additional information
Deceased.
Scientific-Research Institute of Physical and Organic Chemistry, Rostov State University, Rostov-na-Donu 344104. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 5, pp. 640-643, May, 1995. Original article submitted April 20, 1995.
Rights and permissions
About this article
Cite this article
Morkovnik, A.S., Suslov, A.N., Klimov, E.S. et al. Electron transfer as the initiating stage of dehydrogenation of benzimidazolines by the triphenylmethyl cation. Chem Heterocycl Compd 31, 563–566 (1995). https://doi.org/10.1007/BF01166330
Issue Date:
DOI: https://doi.org/10.1007/BF01166330