Summary
Arabinosyl-5-azacytosine (AAC), a new nucleoside antimetabolite, is broadly active in preclinical tumor screening evaluations. To assess the potential for intrathecal use of this drug, we studied the toxicity and pharmacokinetics of intrathecal and intraventricular administration in nonhuman primates.
Four adult male rhesus monkeys were given single 10 mg intrathecal (n=1) or intraventricular (n=3) doses of AAC to determine its acute toxicity and pharmacokinetic parameters. An additional 3 animals were given four weekly 10 mg intrathecal doses to assess the systemic and neurologic toxicity associated with chronic administration.
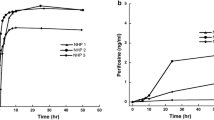
Disappearance from the cerebrospinal fluid (CSF) was biexponential, and CSF clearance was 0.2 ml/min, which exceeds the rate of CSF bulk flow by 5-fold. The peak CSF concentration and area under the concentrationx time curve achieved with the intraventricular administration of 10 mg were one hundred, and fifty fold greater, respectively, than those achieved after an intravenous dose of 200 mg/kg (1500–2400 mg) in prior experiments. No clinically evident neurotoxicity was observed in either the single or the weekly × 4 dose groups. A slight, transient CSF pleocytosis and increased CSF protein was observed. Systemic toxicity was limited to one animal in the weekly × 4 dose group who demonstrated a mild and transient decrease in his peripheral leukocyte count unassociated with a change in his hematocrit or platelet count.
These studies in nonhuman primates demonstrate a clear pharmacokinetic advantage for intrathecal vs systemic administration of AAC. This is demonstrated by a 50-fold greater CSF drug exposure with an intrathecal or intraventricular dose 1/200th of that which can be given systemically. Intrathecal AAC was found to be safe on a weekly dosing schedule. On the basis of these results, human trials evaluating intrathecal AAC are planned.
Similar content being viewed by others
References
Beisler JA, Abbasi MM, Driscoll JA: Synthesis and antitumor activity of 5-azacytosine arabinoside. J Med Chem 22:1230–1234, 1979
Townsend A, Leclerc JM, Dutschman G, Cooney D, Cheng YC: Metabolism of 1-β-D-arabinosyl-5-azacytosine and incorporation into DNA of human T-lymphoblastic cells (Molt-4). Cancer Res 45:3522–3528, 1985
Dalal M, Plowman J, Breitman TR, Schuller HM, Del Campo A, Vistica D, Cooney DA, Johns DG: Arabinofuranosyl-5-azacytosine: antitumor and cytotoxic properties. Cancer Res 46:831–838, 1986
Grem JL, Shoemaker DD, Hoth DF, King SA, Plowman J, Zaharko D, Greishaber CK, Harrison SD, Craddock JA, Leyland-Jones B: Arabinosyl-5-azacytidine: a novel nucleoside entering clinical trials. Invest New Drugs 5:315–328, 1987
Townsend AJ, Cheng Y-C: Sequence-specific effects of ara-5-aza-CTP and ara-CTP on DNA synthesis by purified human DNA polymerasesin vitro: visualization of chain elongation on a defined template. Molec Pharmacol 32:330–339, 1987
Driscoll JS, Johns DG, Plowman J: Comparison of the activity of arabinosyl-5-azacytosine, arabinosyl cytosine, and 5-azacytidine against intracerebrally implanted L1210 leukemia. Invest New Drugs 3:331–334, 1985
Wallace RE, Lindh D, Durr FE: Arabinosyl-5-azacytosine: activity against human tumors in athymis mice. Cancer Chemother Pharmacol 25:117–123, 1989
Surbone A, Ford H, Thomas R, Kelley JA, Ben-Baruch N, Thomas RV, Fine R, Cowan KH: A phase I and pharmacokinetic study of arabinosyl-5-azacytosine. Cancer Res 50:1220–1225, 1990
Heideman RL, Balis FM, McCully C, Poplack DG: Preclinical pharmacology of arabinosyl-5-azacytidine in nonhuman primates. Cancer Res 48:4294–4298, 1988
Heideman RL, Gillespie A, Ford H, Reaman GH, Balis FM, Tan C, Sato J, Ettinger LJ, Packer RJ, Poplack DG: Phase I trial and pharmacokinetic evaluation of Fazarabine in children. Cancer Res 49:5213–5216, 1989
Donehower RC, Karp JE, Burke PJ: Pharmacology and toxicity of high-dose cytarabine by 72-hour continuous infusion. Cancer Treat Rep 70:1059–1065, 1986
Guide for the Care and Use of Laboratory Animals, HEW publication (NIH) 84-23, revised edition. Washington, DC: Department of Health Education and Welfare, 1988
McCully CL, Balis FM, Bacher J, Phillips J, Poplack DG: A rhesus monkey model for continuous infusion of drugs into cerebrospinal fluid. Lab Animal Sci 40:250–255, 1990
Heideman RL, Roth JS, Ford H, Kinnard RD, Litterst CL, Kelley JA: Reverse phase HPLC determination and murine pharmacokinetics of arabinosyl-5-azacytosine. J Liquid Cromatog 12:1613–1633, 1989
Knott GD: MLAB-a mathematical modeling tool. Comput Programs Biomed 10:271–280, 1979
Yamaoka K, Nakagawa T, Uno T: Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharmaceut 6:165–175, 1978
Gibaldi M, Perrier D: Pharmacokinetics, Ed. 2, pp 445–450. New York: Marcael Dekker, 1982
Zimm S, Collins JM, Miser J, Chatterji D, Poplack DG: Cytosine arabinoside cerebrospinal fluid kinetics. Clin Pharm Therap 35:826–830, 1984
Adamson PC, Balis FM, Arndt CA, Holcenberg JS, Narang PK, Murphy RF, Poplack DG: Intrathecal 6-Mercaptopurine: Preclinical pharmacology, Phase I/II trial, and pharmacokinetic study. Cancer Res 51:6079–6083, 1991
Lux WE, Fenstermacher JD: Cerebrospinal fluid formation in ventricles and spinal subarachnoid space of the rhesus monkey. J Neurosurg 42:674–678, 1975
Lopez JA, Beardsley GP, Kirkorian JG, Mortara RW, Agarwal RP: Cerebrospinal fluid and plasma pharmacokinetics of high doses of 1-β-D-Arabinofuranosylcytosine in nonhuman primates. Cancer Res 43:5190–5193, 1983
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heideman, R.L., McCully, C., Balis, F.M. et al. Cerebrospinal fluid pharmacokinetics and toxicology of intraventricular and intrathecal arabinosyl-5-azacytosine (fazarabine, NSC 281272) in the nonhuman primate. Invest New Drugs 11, 135–140 (1993). https://doi.org/10.1007/BF00874147
Issue Date:
DOI: https://doi.org/10.1007/BF00874147