Summary
Systemic chemotherapy with currently available agents has not improved survival for patients with hormone refractory prostate cancer (HRPC), consequently, the evaluation of new agents is warranted. Topotecan is a specific inhibitor of topoisomerase I with broad antitumor activity in preclinical studies. The purpose of this phase II trial was to determine the objective response rate of topotecan administered as a 30 minute infusion for five consecutive days in men with metastatic HRPC. Thirty-four evaluable patients were treated with topotecan 1.1–1.5 mg/m2 as a 30 minute infusion daily for five days, repeated every three weeks until disease progression or unacceptable toxicity. Response was assessed with a combination of standard solid tumor response criteria and the serum prostate specific antigen (PSA) for patients with bidimensionally measurable disease, and by serial measurements of the PSA in patients with bone only (evaluable) disease.
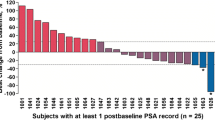
One of 13 patients (7.6%) with measurable soft tissue disease had a PR in nodal sites. Of 21 patients with only osseous metastases, 1 (4.7%) had improvement in bone scan. Six of the 34 evaluable patients (17.6%) had the serum PSA decrease by ≥ 50% and 2 (5.8%) had PSA decreases of ≥ 75%. Toxicity was chiefly hematologic with 66% of patients experiencing Grade 3 or 4 granulocytopenia. Thirty-nine percent of cycles required a delay to allow for hematologic recovery and ten patients required red cell transfusions. Nonhematologic toxicity, mainly nausea and alopecia, was mild. Topotecan administered at this dose and schedule has limited activity in patients with HRPC. Further trials of topo I inibition in HRPC should utilize alternative schedules of topotecan (e.g.), prolonged infusion) or other camptothecin analogs with more potent topo I inhibitory activity.
Similar content being viewed by others
References
Eisenberger MA, Simon R, O'Dwyer PJ, Wittes RE, Friedman MA: A reevaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. J Clin Oncol 3:827–841, 1985
Champoux J: Evidence for an intermediate with a singlestrand break in the reaction catalyzed by the DNA untwisting enzyme. Proc Natl Acad Sci USA 73:3488–3491, 1976
Champoux J: Mechanism of the reaction catalyzed by the DNA untwisting enzyme: Attachment of the enzyme to the 3′-terminus of the nicked DNA. J Mol Biol 118:441–446, 1978
Hsiang Y-H, Hertzberg R, Hect S, Lius LF: Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878, 1985
Burris HA III, Hanauske A, Johnson RK, Marshall MH, Kuhn JG, Hilsenbeck SG, Von Hoff DD: Activity of topotecan, a new topoisomerase I inhibitor, against human tumor colony forming units in vitro. J Natl Can Inst 84:1816–1820, 1992
Wang J: DNA topoisomerases. Annual Rev of Biochem 54:665–697, 1985
Topotecan (SK&F 104864-A) Investigator Brochure. June 1994
Rowinsky EK, Grochow LB, Hendricks CB, Ettinger DS, Forastiere AA, Hurowitz LA, McGuire WP, Sartorius SE, Lubejko BG, Kaufmann SH, Donehower RC: Phase I and pharmacologic study of topotecan: A novel topoisomerase I inhibitor. J Clin Oncol 10:647–656, 1992
Scher HI, Kelly WK: Flutamide withdrawal syndrome: Its impact on clinical trials in hormone refractory prostate cancer. J Clin Oncol 11:1566–1572, 1993
Fishman B, Pasternak S, Wallenstein SL, Houde R, Holland JC, Foley KM: The Memorial Pain Assessment Card: A valid instrument for the evaluation of cancer pain. Cancer 60:1151–1158, 1987
Tannock I, Gospodarowicz M, Meakin W, Panzarella T, Stewart L, Rider W: Treatment of metastatic prostatic cancer with low-dose prednisone. Evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol 7:590–597, 1989
Myers C, Cooper M, Stein C, LaRocca R, Walther MM, Weiss G, Choyke P, Dawson N, Steinberg S, Uhrich MM, Cassidy J, Kohler DR, Trexel J, Lineham WM: Suramin: A novel growth factor antagonist with activity in hormone refractory, metastatic prostate cancer. J Clin Oncol 10:881–889, 1992
Hudes GR, Greenberg R, Krigel RL, Fox S, Scher R, Litwin S, Watts P, Speicher L, Tew K, Comis R: Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol 10:1754–1761, 1992
Pienta KJ, Redman B, Husain M, Cumings G, Esper PS, Appel C, Flaherty LE: Phase II evaluation of oral estramustine and oral etoposide in hormone-refractory adenocarcinoma of the prostate. J Clin Oncol 12:2005–2012, 1994
Sella A, Kilbourn R, Amato R, Buic C, Zukiwski AA, Ellerhorst J, Logothetis CJ: Phase II study of ketoconazole combined with weekly doxorubicin in patients with androgen-independent prostate cancer. J Clin Oncol 12: 683–688, 1994
Kelly WK, Scher HI, Mazumdar M, Valmis V, Schwartz M, Fossa S: Prostate-specific antigen as a measure of disease outcome in metastatic hormone refractory prostate cancer. J Clin Oncol 11:607–615, 1993
Saltz L, Sirott M, Young C, Tong W, Niedzwiecki D, Yao T, Tao T, Trochanowski B, Wright P, Barbasa K, Toomesi F, Kelson D: Phase I clinical and pharmacology study of topotecan given daily for 5 consecutive days to patients with advanced solid tumors, with attempt at dose intensification using recombinant granulocyte colony-stimulating factor. J Natl Cancer Inst 85:1499–1507, 1993
Tanizawa A, Fujimori A, Fujimori Y, Pommier Y: Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J Natl Cancer Inst 86:836–842, 1994
Mattern MR, Mong SM, Bartus HF, Mirabell CK, Crooke ST, Johnson RK: Relationship between the intracellular effects of camptothecin and the inhibition of DNA topoisomerase I in cultured 1210 cells. Cancer Res 47:1793–1798, 1987
Slichenmeyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH: The current status of camptothecin analogs as antitumor agents. J Natl Cancer Inst 85:271–291, 1993
Houghton PJ, Cheshire PJ, Meyers L, Stewart CFR, Synold TW, Houghton JA: Evaluation of 9-Dimethylaminomethyl-10-hydroxycamptothecin (Topotecan) against xenografts derived from adult and childhood tumors. Cancer Chemother Pharmacol 31:229–239, 1992
Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M: DNA topoisomerase-I targeted chemotherapy of human colon cancer in Xenografts. Science 246:1046–1048, 1984
Hochster H, Liebes L, Speyer J, Sorich J, Taubes B, Oratz R, Wernz J, Chachoua A, Raphael B, Vinci RZ, Blum RH: Phase I trial of low-dose continuous topotecan infusion in patients with cancer: An active and welltolerated regimen. J Clin Oncol 12:553–559, 1994
Creemers GJ, Schellens JH, Beijnen JH, Planting AS, Rosing H, de Boer-Dennert M, van der Burg ME, Loos WJ, McDonald M, Stoter G, Verweij J: Bioavailability of oral topotecan, a new topoisomerase I inhibitor. Proceedings of ASCO 13:132, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hudes, G.R., Kosierowski, R., Greenberg, R. et al. Phase II study of topotecan in metastatic hormone-refractory prostate cancer. Invest New Drugs 13, 235–240 (1995). https://doi.org/10.1007/BF00873806
Issue Date:
DOI: https://doi.org/10.1007/BF00873806