Abstract
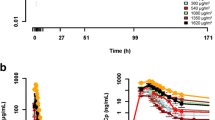
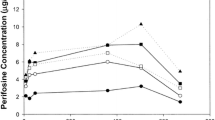
Ilmofosine, an ether lipid derivative of lysophosphatidylcholine has antineoplastic activityin vitro andin vivo. Maximum efficacy in preclinical models is associated with prolonged exposure to the drug. In a Phase I trial of a weekly 2 hour infusion schedule of ilmofosine, a syndrome of lethargy, diminished performance status, and mild hepatotoxicity was dose-limiting at 550 mg/m2. To avoid the higher drug concentrations associated with a brief infusion, a Phase I study of a weekly 24 hour infusional schedule was undertaken in an attempt to maximize dose-intensity. Doses were escalated from 550 to 800 mg/m2. Toxicities included nausea, anorexia, fatigue, and minor elevations of liver function tests. The dose limiting toxicity at 800 mg/ m2 was a syndrome of severe abdominal pain. No neutropenia or thrombocytopenia was observed except in one patient who was found to have a myelodysplastic syndrome, thought not to be related to drug therapy. The more prolonged infusion schedule of ilmofosine did not result in a substantial increase in the tolerable dose.
Similar content being viewed by others
References
Hanauske A, Degen D, Marshall MH, Hilsenbeck SG, McPhillips JJ, Von Hoff DD: Preclinical activity of ilmofosine against human tumor colony forming unitsin vitro. Anti-Cancer Drugs 3:43–46, 1992
Noseda A, Godwin PL, Modest EJ: Effect of antineoplastic ether lipids on model and biologic membranes. Biochim Biophys Acta 945:92–100, 1988
Shoji M, Raynor RL, Berdel WE, Vogler WE, Kuo JF: Effects of throether phospholipid BM41.440 on protein kinase C and phorbolester-induced differentiation of human leukemia HE60 and KG-1 cells. Cancer Res 48: 6669–6679, 1988
Herrmann DBJ, Pahlke W, Opitz H-G, Bicker U:In vivo antitumor activity of Ilmofosine. Cancer Treatment Reviews 17:247–252, 1990
Herrmann DBJ, Opitz H-G, Munder PG: Antitumor activity of ilmofosine (BM41.4400) in the 3 Lewis-lung carcinoma model. Lipids 26:1431–1436, 1991
Ilmofosine Clinical Brochure. Boehringer Mannheim, 1992, p 6
Rodriguez G, Havlin K, Burris H, Wall J, Schaffer D, Smith L, Kalter S, Brown T, Cagnola J, Weiss G, Kneuper Hall R, Von Hoff D: Phase I clinical trial of ilmofosine, a novel antitumor agent. Proc Amer Soc Clin Oncol 10:114 (Abs 326), 1991
Giantonio BJ, Derry C, McAleer C, Schilder R, McPhillips J, O'Dwyer PJ: Phase I study of ilmofosine administered by two schedules in patients with advanced cancer. Proc Amer Assoc Cancer Res 35:A2220, 1994
WHO Handbook for Reporting Results of Cancer Treatment. WHO offset publication. World Health Organization, Geneva, Switzerland, 1979
Miller AB, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47:207–214, 1981
Workman P: The cell membrane and cell signals: New targets for novel anticancer drugs. Annals of Oncology 1:100–111, 1990
Koeller J, Rodriguez G, Smith L, Eckardt J, Shaffer D, Weiss G, Higashi L, McPhillips J, Von Hoff D: Phase I study of ilmofosine given as a 120 hour continuous infusion in patients with solid tumors. Proc Am Soc Clin Oncol 12:A421, 1993
Berdel WE, Fink V, Rastetler J: Clinical Phase I pilot study of the alkyl lysophospholipid derivative ET-18-OCH3. Lipids 22:967–969, 1987
Hermann DB, Neumann HA, Berdel WE, Heim ME, Fromm M, Boerner D, Bicker V: Phase I trial of the thioether phospholipid BM41.440 in cancer patients. Lipids 22:962–966, 1987
Guenther I, Drings P, Khanavkar B, Ulbuch F, Gatzemeier U, Nordstroem R: Alkyl-lysophospholipid ET 18 OCH3 in the treatment of advanced non-small cell carcinoma of the lung. J Chemother Infect Dis Malignancies (Suppl 1):A12, 1989
Rodriguez G, Havlin K, Burris H,et al: Phase I clinical trial of ilmofosine, a novel antitumor agent. Proc Ann Meet Am Soc Clin Oncol 10:A326, 1991
Danhauser-Riedl S, Drozd A, Zafferani M, Bruntsch V, Paukert M, Sindermann H, Prauer HW, Siewert JR, Rastetter J, Berdel WE: Phase I study of weekly oral miltefosine (Hexadecyl-Phosphocolini; MIL) in cancer patients. Proc Ann Meet Am Soc Clin Oncol 10:A304, 1991
Winkelmann M, Ebeling K, Strohmeyer G, Hottenrott G, Mechl Z, Beyes W, Scholten T, Westerhausen M, Schlinok G, Sterz R: Treatment results of the thioether lipid ilmofosine in patients with malignant tumors. J Cancer Res and Clinical Oncology 118:403–407, 1992
Berdel WG, Becher R, Edler K, Bremer K, Essers V, Bruntsch V, Zafferani M, Klee M, Bachmann P, Westerhausen M: Phase II trial of oral miltefosin (MIL) in patients with non-small cell lung cancer. Proc Ann Meet Am Assoc Cancer Res 33:A2482, 1992
Hofmann J, Ueberall F, Pesch L, Maly K, Hermann DB, Grunicke H: Synergistic enhancement of the antiproliferative activity of cis-diammine-dichloroplatinum II by the ether lipid analog BM 41440, an inhibitor of protein kinase C. Lipids 24:312–317, 1989
Andreesen R, Modolell M, Weltzienb HU, Eibl H, Common HH, Lohr W, Munder PG: Selective destruction of human leukemic cells by alkyl-lysophospholipids. Cancer Res 38:3894–3899, 1978
Andreesen R, Modolell M, Munder PG: Selective sensitivity of chronic myelogenous leukemia cell population to alkyl-lysophospholipids. Blood 54:519–523, 1979
Vogler WR: Bone marrow purging in acute leukemia with alkyl-lysophospholipids: a new family of anticancer drugs. Leukemia and lymphoma 13:53–60, 1994
Okamoto S, Olson AC, Vogler WR, Winton EF: Purging leukemic cells from simulated human remission marrow with alkyl-lysophospholipid. Blood 69:1381–1387, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
von Mehren, M., Giantonio, B.J., McAleer, C. et al. Phase I trial of ilmofosine as a 24 hour infusion weekly. Invest New Drugs 13, 205–210 (1995). https://doi.org/10.1007/BF00873801
Issue Date:
DOI: https://doi.org/10.1007/BF00873801