Summary
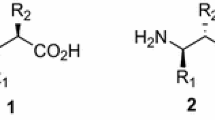
Methods for the synthesis of racemic and optically active title compounds are presented. Key step of these four-step procedures is the alkylation with 1-bromo-2-fluoroalkanes of glycine-ester-derived imines in anhydrous medium using lithium diisopropylamide as a base at low temperature or phase transfer catalyzed alkylation with 50% NaOH and triethylbenzylammoniumchloride as the phase transfer catalyst, respectively. Subsequent three-step deprotection gave the free acids in 13–33% overall yield. Deracemization ofγ-fluoro-α-aminobutyric acid methyl and ethyl esters withα-chymotrypsin was shown to give the (−)-enantiomers of the esters and (+)-γ-fluoro-α-aminobutyric acid in >98% ee, while from thetert-butylester the opposite stereochemical result was observed giving the (−)-acid with 88% ee. Optically activeγ-fluoro-α-amino acids were synthesized alternatively by phase transfer catalysis with N-benzyl-cinchonium chloride or using an auxiliary-directed asymmetric alkylation of the imine derived from (R)-(+)-camphor or (R)-(+)-2-hydroxypinan-3-one. These processes gave different enantiomers ofγ-fluoro-α-aminobutyric acid via a monomeric lithium enolate in the first or a dimeric lithium enolate in the second case, respectively. The enantiomeric excess can be improved by lithium/magnesium exchange.
Similar content being viewed by others
References
Alekseeva LV, Lundin BN, Burdé NL (1967) Synthesis and investigation of compounds with potential biological activity. Zh Obshch Khim 37: 1754–55 (J Gen Chem (USSR) (engl Transl) 37: 1671–1672)
Alvernhe G, Laurent A, Haufe G (1987) Triethylamine trishydrofluoride [(C2H5)3N·3HF]: a highly versatile source of fluoride ion for the halofluorination of alkenes. Synthesis 562–564
Bergman ED, Chun-Hsu L (1973) Organic fluorine compounds. Part 46.γ-Fluoroglutamic acid and fluorofolic acid. Synthesis: 44–46
Bory S, Dubois J, Gaudry M, Marquet A, Lacombe L, Weinstein S (1984) Resolution ofγ-methyl andγ-fluoroglutamic acids. Lack of stereoselectivity of Leucine Aminopeptidase with L-Leucyl-L-erythro-γ-substituted glutamates. J Chem Soc, Perkin Trans 1: 475–480
Buchanan RL, Dean FH, Pattison FLM (1962)γ-Fluoroglutamic acid. Can J Chem 40: 1571–1575
Butina D, Hudlický M (1980) The synthesis ofγ-fluoroisoleucine. J Fluorine Chem 16: 301–323
Cavalleri B, Bellasio E, Testa E (1966) Indagine su composti organici fluorurati a potenziale attività biologica. Derivati fluorurati dell'acidoα-amino eα,α′-diaminopimelico. Gazz Chim Ital 96: 253–263
Gershon H, Shanks L, Clarke DD (1978) Amino acid analogs, IV. 4-Fluoroisoleucine. J Pharm Sci 67: 715–717
Haufe G, Kröger S (1995) Synthesis ofγ-fluoro-α-amino acids. 4th International Congress on Amino Acids, Vienna, August 7–11th.
Haufe G, Alvernhe G, Laurent A, Ernet T, Goj O, Kröger S, Sattler A (1996) Bromofluorination of alkenes. Organic Syntheses (submitted)
Hudlický M (1960) The synthesis ofγ-fluoroglutamic acid. Tetrahedron Lett 14: 21–22
Hudlický M (1961) Organic compounds of fluorine, II. Fluorinated amino acids. Coll Czech Chem Commun 26: 1414–1421
Hudlický M, Merola JS (1990) New stereospecific syntheses and X-ray diffraction structures of (−)-d-erythro- and (+)-l-threo-4-fluoroglutamic acid. Tetrahedron Lett 31: 7403–7406
Hudlický M (1993) Stereospecific syntheses of all four stereoisomers of 4-fluroglutamic acid. J Fluorine Chem 60: 193–210
Kröger S (1996) Synthese fluorierter Aminosäuren. PhD Dissertation, Münster
Kukhar' VP, Soloshonok VA (1995) Fluorine-containing amino acids. Synthesis and properties. Wiley, Chilchester
Lettré H, Wölcke U (1967) Fluor-Derivate biogener aliphatischer Aminosäuren. Liebigs Ann Chem 708: 75–85
McIntosh JM, Leavitt RK, Mishra P, Cassidy KC, Drake JE, Chadha R (1988) Diastereoselective alkylation guided by electrophile-nucleophileπ-interactions. J Org Chem 53: 1947–1952
O'Donnel MJ, Boniece JM, Earp SE (1978) The synthesis of amino acids by phasetransfer reactions. Tetrahedron Lett 30: 2641–2644
O'Donnel MJ, Bennett WD, Wu S (1989) The stereoselective synthesis ofα-amino acids by phase-transfer catalysis. J Am Chem Soc 111: 2353–2355
Oguri T, Kawai N, Shioiri T, Yamada S-I (1978) Amino acids and peptides 29. A new efficient asymmetric synthesis ofα-amino acid derivatives with recycle of a chiral reagent-asymmetric alkylation of chiral Schiff base from glycin. Chem Pharm Bull (Jpn) 26: 803–808
Papageorgiou C, Borer X, French RR (1994) Calcineurin has a very tightbinding pocket for the chain of residue 4 to cyclosporin. Bioorg Med Chem Lett 4: 267–272
Raasch MS (1958) 5-Fluoronorvaline and 6-fluoronorleucine. J Org Chem 23: 1567–1568
Schöllkopf U (1993) Enantioselective synthesis of nonproteinogenic amino acids. Topics Current Chemistry 109: 65–84
Solladié-Cavallo A, Simon MC (1989) Enantioselective synthesis of optically pure natural S(+) or unnatural R(−) DABA. Tetrahedron Lett 30: 6011–6014
Solladié-Cavallo A, Simon-Wermeister MC, Schwarz J (1993) Diastereoselective monoalkylation of lithium and potassium enolates of a chiral imine of ethyl glycinate: the role of added salts. Organometallics 12: 3743–3747
Tolman V (1993) Chemistry of 4-fluorogluatmic acid. Part 1. A critical survey of its syntheses: an attempt to optimize reaction conditions for large-scale preparation. J Fluorine Chem 60: 179–183
Tolman V (1995) Syntheses of fluorine-containing amino acids by methods of classical amino acid chemistry. In: Kukhar' VP, Soloshonok VA (eds) Fluorine-containing amino acids. Synthesis and properties. Wiley, Chichester, pp 1–70
Tolman V, Vereš K (1966) Potential antimetabolites derived from 4-fluoroglutamic acid. Tetrahedron Lett 3909–3912
Tolman V, Vereš K (1967) Synthesis of certain monofluorinated aliphatic amino acids. Coll Czech Chem Commun 32: 4460–4469
Tolman V, Špronglová P (1983) Synthesis of 2-fluoropenoic acid derivatives. Coll Czech Chem Commun 48: 319–326
Unkeless JC, Goldman P (1970) Fluorinatedγ-aminobutyric acid. Enzymatic synthesis and biological activity of a potentially useful analogue. Mol Pharmacol 6: 46–53
Unkeless JC, Goldman P (1971) The diastereomers ofγ-fluorogluatmate: complementary structural analogues. Mol Pharmacol 7: 293–300
Yamada S-I, Oguri T, Shioiri T (1976) Asymmetric synthesis ofα-amino acid derivatives by alkylation of a chiral Schiff base. J Chem Soc, Chem Commun: 136–137
Yaozhong J, Guilan L, Changyou Z, Huri P, Lanjun W, Aiqiao M (1991) Asymmetric synthesis XIII: the stereocontrolled synthesis of (R)-α-amino acids via a double chiral induction. Synth Commun 21: 1087–1090
Yarovenko NN, Raksha MA (1959) Fluorination by means ofα-fluorinated amines. J Gen Chem USSR (engl Transl) 29: 2125–2128
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haufe, G., Kröger, S. Syntheses ofγ-fluoro-α-amino acids. Amino Acids 11, 409–424 (1996). https://doi.org/10.1007/BF00807945
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807945