Summary
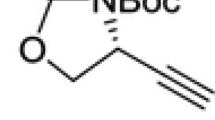
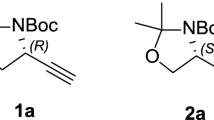
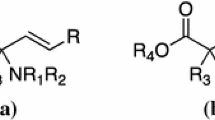
Ethynyl glycine is a naturally occurring unusualα-amino acid. Its known chemical and biological properties are summarized in the first part of this review. The second part is an overview on racemic syntheses of ethynyl glycine and otherβ,γ-alkynylα-amino acid derivatives, including patent data. These small polyfunctional compounds revealed as being very labile and the synthesis of mainly fully or partially protected forms seemed to have been actually performed. The last part deals with the approaches to the enantioselective synthesis ofβ,γ-alkynylα-amino acids derivatives, and details the only satisfactory strategy that has led to optically activeβ,γ-alkynylα-amino acids derivatives up to now.
Similar content being viewed by others
References
Abdulganeeva SA, Erzhanov KB (1991) Acetylenic amino acids. Russ Chem Rev 60: 676–688
Abeles RH, Maycock AL (1976) Suicide enzyme inactivators. Acc Chem Res 9: 313–319
Abeles RH (1980) Suicide enzyme inactivators and others. Pure Appl Chem 53: 149–160
Abeles RH (1983) Suicide enzyme inactivators. Chem Eng News 61: 48–56
Agouridas C, Tessot N, Martel A (1990) Nouveaux dérivés insaturés de l'acide 2,6-amino heptanedioique, leur procédé de préparation et leur application comme médicaments. Eur P 0394118
Angst C (1987) Stereoselective synthesis ofβ,γ-unsaturated amino acids. Pure Appl Chem 59: 373–380
Beaulieu PL, Duceppe JS, Johnson C (1991) Synthesis of chiral vinyl glycines. J Org Chem 56: 4196–4204
Bey P (1989) Mechanism-based inhibitors: fifteen years later. XXIVèmes rencontres internationales de chimie thérapeutique (1988) Strasbourg, France. Société de chimie thérapeutique Pub. 16ème série, pp 111–122
Bijoy P, Subba-Rao GSR (1993) An unusual C-C bond cleavage with chromium-VI reagents: oxidation of primary alcohols to ketones. Synth Commun 23: 2701–2708
Bowlin TL (1992) 5-Amine substituted adenosine analogs as immunosuppressants. Eur P 0472181
Branquet E, Durand P, Vo-Quang L, Le Goffic F (1993) A straightforward synthesis of N-tert-butoxycarbonyl serinate acetonide methyl ester. Synth Commun 23: 153–156
Cainelli G, Cardillo G (1984) Chromium oxidation in organic synthesis. Springer, Berlin Heidelberg New York Tokyo, pp 118–151, pp 204–216
Casara P, Danzin C (1990) S-adenosylmethionine decarboxylase inhibitors. Eur P 0351475
Casara P, Metcalf B (1978) Trimethylsilylacetylene-N-carboethoxy glycinate dianion — a general synthon forα-acetylenicα-amino acids. Tetrahedron Lett: 1581–1584
Casara PJ, Jung M, Metcalf BW (1978) Lower alkyl 2-tri-(lower)alkylsilylacetylene-N-carbethoxyglycinates and process for using same. US P 4088667
Castelhano AL, Horne S, Taylor GJ, Billedeau R, Krantz A (1988) Synthesis ofα-amino acids withβ,γ-unsaturated side chains. Tetrahedron 44: 5451–5466
Colson PJ, Hegedus LS (1993) Asymmetric synthesis ofα-alkyl-α-amino acids from chromium-carbene-complex-derivedβ-lactams. J Org Chem 58: 5918–5924
Danzin C, Casara P, Claverie N, Metcalf BW (1981)α-Ethynyl andα-vinyl analogues of ornithine as enzyme-activated inhibitors of mammalian ornithine decarboxylase. J Med Chem 24: 16–20
Duthaler RO (1991) Synthesis ofβ,γ-unsaturated D-α-amino acids from L-cysteine. Angew Chem Int Ed Engl 30: 705–707
Duthaler RO (1992) Partialsynthese von ungewöhnlichen Aminosäuren aus Serin oder Cystein. GIT Fachz Lab 36: 479–488
Duthaler RO (1994) Recent developments in the stereoselective synthesis ofα-amino acids. Tetrahedron 50: 1539–1650
Duthaler RO, Hafner A (1994) New tools for stereoselective synthesis: A.(L)-cystein as (D)-amino acid synthon. B. Chiral titanium reagents. In: Makriyannis A, Castagnoli N (eds) Proceedings of the 5th Cyprus conference on new methods in drug design (1992). JR Prous Science Publishers SA
Garner P (1984) Stereocontrolled addition to a penaldic acid equivalent: an asymmetric synthesis ofthreo-β-hydroxy-L-glutamic acid. Tetrahedron Lett 25: 5855–5858
Garner P, Park JM (1987) The synthesis and configurational stability of differentially protectedβ-hydroxy-α-amino aldehyde. J Org Chem 52: 2361–2364
Garner P, Park JM (1992) 1,1-dimethyl (S)- or (R)-4-formyl-2,2-dimethyl-3-oxazolidine carboxylate: a useful serinal derivative. Org Synth 70: 18–28
Haines AH (1985) Best synthetic methods: methods for the oxidation of organic compounds: alkanes, alkenes, alkynes and arenes. Academic Press, London, pp 153–172
Haines AH (1988) Best synthetic methods: methods for the oxidation of organic compounds: alcohols, alcohols derivatives, alkyl halides, nitroalkanes, alkyl azides, carbonyl compounds, hydroxyarenes and aminoarenes. Academic Press, London, pp 148–165
Havlicek L, Hanus J (1991) Syntheses ofβ,γ-unsaturatedα-amino acids. Collect Czech Chem Commun 56: 1365–1399
Holland BC, Gilman NW (1974) An improved procedure for the oxidation of alkynols to alkynoic acids. Synth Commun 4: 203–210
John RA (1980) Enzyme inhibitors as drugs: enzyme-induced inactivation of pyridoxal phosphate-dependent enzymes: approaches to the design of specific inhibitors. In: Sandler M (ed) Macmillan Press ltd, London Basingstoke, pp 73–93
Jung MJ (1985) Chemistry and biochemistry of the amino acids. In: Barret GC (ed) Chapman and Hall, London New York, pp 227–245
Jung MJ, Palfreyman MG, Ribereau-Gayon G, Bey P, Metcalf BW, Koch-Weser J, Sjoerdsma A (1979) Drug action and design: mechanism-based enzyme inhibitors: effects of enzyme-activated irreversible inhibitors of aromatic amino acids decarboxylase on endogenous synthesis of biogenic amines. In: Kalman T (ed) Proceedings of the twentieth annual medicinal chemistry symposium. Amherst, New York, USA, May 1979. Developments in biochemistry, vol 6. Elsevier/North-Holland, New York Amsterdam Oxford, pp 131–144
Kuroda Y, Okuhara M, Goto T, Iguchi E, Kohsaka M, Aoki H, Imanaka H (1980a) FR-900130, a novel amino acid antibiotic I. discovery, taxonomy, isolation, and properties. J Antibiotics 33: 125–131
Kuroda Y, Okuhara M, Goto T, Kohsaka M, Aoki H, Imanaka H (1980b) FR-900130, a novel amino acid antibiotic II. isolation and structure elucidaion of the acetyl derivative of FR-900130. J Antibiotics 33: 132–136
Li M, Johnson ME (1995) Oxidation of certain 4-substituted phenethyl alcohols with collins reagents: on the mechanism of a carbon-carbon bond cleavage. Synth Commun 25: 533–537
Manfré F, Kern JM, Biellman JF (1992) Syntheses of proline analogues as potential mechanism-based inhibitors of proline dehydrogenase: 4-methylene-L-, (E)- and (Z)-4-(fluoromethylene)-L-,cis- andtrans-5-ethynyl-(±)-, andcis- andtrans-5-vinyl-L-proline. J Org Chem 57: 2060–2065
Maycock AL, Aster SD, Patchett AA (1979) Drug action and design: mechanism-based enzyme inhibitors: suicide inactivation of decarboxylases. In: Kalman T (ed) Proceedings of the twentieth annual medicinal chemistry symposium. Amherst, New York, USA, May 1979. Developments in biochemistry, vol 6. Elsevier/North-Holland, New York Amsterdam Oxford, pp 115–129
Meffre P, Durand P, Branquet E, Le Goffic F (1994) A straightforward synthesis of N-Boc-L-serinal and N-Boc-L-threoninal acetonides. Synth Commun 24: 2147–2152
Meffre P, Gauzy L, Perdigues C, Desanges-Levecque F, Branquet E, Durand P, Le Goffic F (1995) En route to optically active ethynyl glycine derivatives. Tetrahedron Lett 36: 877–880
Meffre P, Gauzy L, Branquet E, Durand P, Le Goffic F (1996) Synthesis of optically activeβ,γ-alkynylglycine derivatives. Tetrahedron (in press)
Mehmandoust M, Petit Y, Larchevêque M (1992) Synthesis of (E)-β,γ-unsaturatedα-amino acids by rearrangement of allyltrichloroacetimidates. Tetrahedon Lett 33: 4313–4316
Metcalf BW, Casara P (1979) Synthetic access toα-substituted prop-2-ynylamines andα-acetylenic amino acids via the t-butyl N-trimethylsilyprop-2-ynylcarbamate dianion. J Chem Soc Chem Comm: 119–120
Metcalf BW, Jung M (1979)α-Acetylenic derivatives ofα-amino acids. US P 4133964
Metcalf B, Sjoerdsma A (1979) Drug action and design: mechanism-based enzyme inhibitors: the microscopic reversibility principle in enzyme inhibition. In: Kalman T (ed) Proceedings of the twentieth annual medicinal chemistry symposium. Amherst, New York, USA, May 1979. Developments in biochemistry, vol 6. Elsevier/North-Holland, New York Amsterdam Oxford, pp 61–73
Metcalf BW, Jung M (1980)α-Acetylenic derivatives ofα-amino acids. US P 4190586
Monroe RF, Lowes FJ, Foster GL, Oakes BD (1963) Propargyl compounds as corrosion inhibitors. US P 3079345
Mosher HS, Dale JA (1973) Nuclear magnetic resonance enantiomer reagents. Configurational correlationsvia nuclear magnetic resonance chemical shifts of diastereomeric mandelate, O-methylmandelate, andα-methoxy-α-trifluoromethylphenylacetate (MTPA) esters. J Amer Chem Soc 95: 512–519
Nagata C, Yamaguchi T (1979) Molecular orbital study on the reaction mechanism of irreversible enzyme inhibitors. J Med Chem 22: 13–17
Ohira S (1989) Methanolysis of dimethyl (1-diazo-2-oxopropyl)phosphonate: generation of dimethyl(diazomethyl)phosphonate and reaction with carbonyl compounds. Synth Commun 19: 561–564
Rando RR (1974a)β,γ-Unsaturated amino acids as irreversible enzyme inhibitors. Nature 250: 586–587
Rando RR (1974b) Chemistry and enzymology of kcat inhibitors. Science 185: 320–324
Rando RR (1974c) Irreversible inhibition of aspartate aminotransferase by 2-amino-3-butenoic acid. Biochemistry 13: 3859–3863
Rando RR (1975) Mechanisms of action of naturally occuring irreversible enzyme inhibitors. Acc Chem Res 8: 281–288
Rando RR (1976) Mechanism of the irreversible inhibition of aspartate aminotransferase by the bacterial toxin L-2-amino-4-methoxy-trans-3-butenoic acid. J Biol Chem 251: 3306–3312
Rando RR (1978) Enzyme-activated irreversible inhibitors: principles of catalytic enzyme inhibition. In: Seiler N, Jung MJ, Koch-Weser J (eds) Elsevier/North-Holland Biomedical Press, Amsterdam New York Oxford, pp 13–26
Rando RR (1979) Drug action and design: mechanism-based enzyme inhibitors: natural and synthetic kcat-inhibitors of transaminases and decarboxylases. In: Kalman T (ed) Proceedings of the twentieth annual medicinal chemistry symposium. Amherst, New York, USA, May 1979. Developments in biochemistry, vol 6. Elsevier/North-Holland, New York Amsterdam Oxford, pp 47–60
Rando RR (1984) Mechanism-based enzyme inactivators. Pharmacol Rev 84: 111–142
Reginato G, Mordini A, Degl'Innocenti A, Caracciolo M (1995) Stereoselective synthesis of (R)-(−)-2,2-dimethyl-3-t-butoxycarbonyl-4-ethynyl-oxazolidine: a chiral building block for the synthesis of a new class of substituted alkynes. Tetrahedron Lett 36: 8275–8278
Schöllkopf U, Westphalen KO, Schröder J, Horn K (1988) Studies on the acylation of lithiated bislactim ethers of cyclo(-L-Val-Ala-) and cyclo(-L-Val-Gly); asymmetric synthesis of (R)-α-alkenyl and (R)-α-ethinyl alanine methyl esters by the bislactim ether method. Liebigs Ann Chem 88: 781–786
Sisido K, Hirowatari N, Tamura H, Kobata H, Takagisi H, Isida T (1970) Syntheses of all of the racemic diastereoisomers of phytosphingosine. J Org Chem 35: 350–353
Stanley MS (1992) Orthogonally protected N-(carboxymethyl)-L-2,3-diaminopropanoic acids and O-(carboxymethyl)-L-serines for solid-phase peptide synthesis. J Org Chem 57: 6421–6430
Stark GR, Bartlett PA (1983) Design and use of potent, specific enzyme inhibitors. Pharm Ther 23: 45–78
Walsh C (1982) Suicide substrate: mechanism-based enzyme inactivators. Tetrahedron 38: 871–909
Williams RM (1989) Synthesis of optically activeα-amino acids. Pergamon Press, Oxford
Williams RM, Zhai W (1988) Versatile, strereocontrolled, asymmetric synthesis of Evinyl glycine derivatives. Tetrahedron 44: 5425–5430
Williams RM, Aldous DJ, Aldous SC (1990a) General synthesis ofβ,γ-alkynylglycine derivatives. J Org Chem 55: 4657–4663
Williams RM, Aldous DJ, Aldous SC (1990b) Synthesis of ethynyl glycine (FR-900130). J Chem Soc Perkin Trans I: 171–172
Zhai D, Zhai W, Williams (1988) Alkynylation of mixed acetals with organotin acetylides. J Am Chem Soc 110: 2501–2505
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meffre, P., Le Goffic, F. β,γ-Alkynylα-amino acids: a synthetic challenge. Amino Acids 11, 313–328 (1996). https://doi.org/10.1007/BF00807939
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807939