Summary
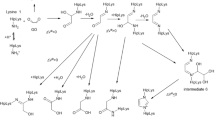
The nature of the products arising from a 10 days, sterile incubation at 37°C and pH 7.2 of a 1:1 mixture of N-α-(p-tosyl)-lysine-methylesterhydrochloride and anhydrous D-glucose was investigated by fast atom bombardment mass spectrometry and1H and13C nuclear magnetic resonance spectroscopies. Differently to the reactivity usually described on the basis of other analytical techniques, FAB mass spectrometric measurements indicate the occurrence of the reaction of protected lysine with more than one D-glucose molecule.
Similar content being viewed by others
References
Arpino P (1990) Combined liquid chromatography mass spectrometry. Part II. Tecnhiques and mechanisms of thermospray. Mass Spectrom Rev 9: 631–669
Bailey AJ, Kent MJ (1988) Non-enzymatic glycosylation of fibrous and basement membrane. In: Baynes JW, Monnier VM (eds) The Maillard reaction in aging, diabetes and nutrition. Alan R. Liss, New York, pp 109–122
Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, Thorpe SR (1988) The Amadori product on protein: structure and reactions. In: Baynes JW, Monnier VM (eds) The Maillard reaction in aging, diabetes and nutrition. Alan R. Liss, New York, pp 43–67
Barber M, Bordoli RS, Sedwick RD, Tyler AN (1981) Fast atom bombardment as ion source in mass spectrometry. Nature 293: 270–275
Berbier C, Gagnaire D, Vottero P (1968) Études stéréochimiques en série tétrahydrofurannique. VI. Étude par RMN des dérivés du dihydro-2,5 et du tétrahydrofuranne. Étude théorique des couplages1JHH cis et trans. Bull Soc Chim Fr 6: 2330–2338
Brownlee M, Vlassara H, Cerami A (1984) Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med 101: 527–537
Batterham TJ (1973) NMR spectra of simple heterocycles, chapt 5. John Wiley & Sons, Malabar, Florida
Cooks RG (1978) Collision spectroscopy. Plenum Press, New York
Coxon B (1983) Two-dimensional J-resolved proton nuclear magnetic resonance spectrometry of hydroxyl-coupledα- andβ-D-glucose. Anal Chem 55: 2361–2368
Eichner K, Reutter M, Wittmann R (1990) Detection of Maillard reaction intermediates by high pressure liquid chromatography (HPLC) and gas chromatography. In: Finot PA, Aeshbacher HU, Hurrell RF, Liardon R (eds) The Maillard reaction in food processing, human nutrition and physiology, Birkäuser, Basel, pp 63–77
Lapolla A, Gerhardinger C, Crepaldi G, Fedele D, Palumbo M, Dalzoppo D, Porter CJ, Ghezzo E, Seraglia R, Traldi P (1991) Mass spectrometric approaches in structural identification of the reaction products arising from the interaction between glucose and lysine. Talanta 38: 405–412
Ledl F (1990) Chemical pathways of the Maillard reaction. In: Finost PA, Aeshbacher HU, Hurrell RF, Liardon R (eds) The Maillard reaction in food processing, human nutrition and physiology. Alan R. Liss, New York, pp 19–42
Ledl F, Severin T (1982) Formation of coloured compounds from hexoses. Z Lebensm Forsch 175: 262–265
Maillard LC (1916) Synthèse des materies huniques par action des acid aminès sur le sucres reducteurs. Ann Chim Ser 9: 258–268
Monnier VM, Cerami A (1982) Nonenzymatic glycosylation and browning of proteins in diabetes. Clin Endocrinol Metab 11: 431–452
Monnier VM (1988) Toward a Maillard reaction theory of aging. In: Baynes JW, Monnier VM (eds) The Maillard reaction in aging, diabetes and nutrition. Alan R. Liss, New York, pp 1–22
Morgan RP, Beynon JH, Bateman RH, Green BN (1978) The MM-ZAB2F double focussing mass spectrometer and MIKE spectrometer. Int J Mass Spectrom Ion Phys 28: 171–191
Reynolds TM (1965) Chemistry of non-enzymatic browning. Adv Food Res 14: 167–283
Suarez G (1988) Nonenzymatic browning of proteins and the sorbitol pathway. In: Baynes JW, Monnier VM (eds) The Maillard reaction in aging, diabetes and nutrition. Alan R. Liss, New York, pp 141–162
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lapolla, A., Gerhardinger, C., Baldo, L. et al. The lysine glycation 1. A preliminary investigation on the products arising from the reaction of protected lysine and D-glucose. Amino Acids 5, 389–401 (1993). https://doi.org/10.1007/BF00806957
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806957