Summary
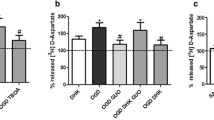
The present study investigates the effects of phenylsuccinate (PS), an inhibitor of the mitochondrial ketodicarboxylate carrier (KCC), on release ofγ-aminobutyric acid (GABA), glutamate (Glu), glutamine (Gln), and glycine (Gly), induced by potassium chloride (KCl) and by cardiac arrest caused by a halothane overdose. Microdialysates were collected from the hippocampus of anaesthetized rats, and analyzed by HPLC. Continuous perfusion of 50 mM PS through the dialysis probe, reduces release of GABA induced by KCl (50 mM for 10 min through the dialysis probe) by up to 72%. In addition, PS abolished KCl-induced release of Glu. Release of GABA during cardiac arrest was not reduced by PS, whereas PS reduced release of Glu in the early stage of cardiac arrest. PS furthermore increased the basal level of Gln, and reversed a decrease of Gln induced by cardiac arrest.
It is proposed that the KCC is present in GABA'ergic neurons of the rat hippocampus, and that GABA, released by KCl, can be synthesized in a KCC dependent manner. It is also suggested that ischemia-induced release of GABA, to some extent, has a non-transmitter origin. The results furthermore indicate that uptake of Gln into GABA'ergic and Glu'ergic neurons is not regulated by simple demand mechanisms.
Similar content being viewed by others
Abbreviations
- PS:
-
phenylsuccinate
- KCC:
-
ketodicarboxylate carrier
- GABA:
-
γ-aminobutyric acid
- Glu:
-
glutamate
- Gln:
-
glutamine
- Gly:
-
glycine
- α-KG:
-
α-ketoglutarate
- Mal:
-
malate
- KRB-buffer:
-
Krebs-Ringer bicarbonate-buffer
- HPLC:
-
high pressure liquid chromatography
References
Arai T, Aoki M, Murakawa M, Nakao S, Mori K, Kurihara N (1990) The effects of halothane on the contents of putative transmitter amino acids in whole rat brain. Neurosci Lett 117: 353–357
Benveniste H, Diemer NH (1987) Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol 74: 234–238
Benveniste H, Drejer J, Schousboe A, Diemer NH (1984) Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 43: 1369–1374
Besson MJ, Gauchy C, Kemel ML, Glowinski J (1981) In vivo release of3H-GABA synthesized from3H-glutamine in the substantia nigra and the pallido entopeduncular nuclei in the cat. Adv Biochem Psychopharmacol 30: 95–103
Brainard JR, Kyner E, Rosenberg GA (1989)13C nuclear magnetic resonance evidence for y-aminobutyric acid formation via pyruvate carboxylase in the brain: A metabolic basis for compartmentation. J Neurochem 53: 1285–1292
Christensen T, Bruhn T, Diemer NH, Schousboe A (1991) Effect of phenylsuccinate on potassium- and ischemia-induced release of glutamate in rat hippocampus monitored by microdialysis. Neurosci Lett 134: 71–74
Collin AK, Ungerstedt U (1988) Microdialysis. User's guide, 4th edn. Repro Print AB, Stockholm
Fonnum F (1981) The turnover of transmitter amino acids with special reference to GABA. In: Pycock CJ, Taberner PV (eds) Central neurotransmitter turnover. University Park Press, Baltimore, pp 105–124
Hertz L, Schousboe A (1987) Primary cultures of GABAergic and glutamatergic neurons as model systems to study neurotransmitter function. I. Differentiated cells. In: Vernadakis A, Privat A, Lauder JM, Timiras PS, Giacobini E (eds) Model systems of development and aging of the central nervous system. Mass, Nijjhoff, pp 19–31
Hertz L, Peng L, Westergård N, Yudkoff M, Schousboe A (1992) Neuronal-astrocytic interactions in metabolism of transmitter amino acids of the glutamate family. In: Schousboe A, Diemer NH, Kofod H (eds) Drug research related to neuroactive amino acids. Alfred Benzon symposium 32. Munksgaard, Copenhagen, pp 30–50
Kamisaki Y, Inagaki S, Tohyama M, Horio Y, Wada H (1984) Immunocytochemical localization of cytosolic and mitochondrial glutamic oxaloacetic transaminase isozymes in rat brain. Brain Res 297: 363–368
Katayama Y, Kawamata T, Tamura T, Hovda DA, Becker DP, Tsubokawa T (1991) Calcium-dependent glutamate release concominant with massive potassium flux during cerebral ischemia in vivo. Brain Res 558: 136–140
Kauppinen RA, McMahon HT, Nicholls DG (1988) Ca2+-dependent and Ca2+-independent glutamate release, energy status and cytosolic free Ca2+ concentration in isolated nerve terminals following metabolic inhibition: Possible relevance to hypoglycaemia and anoxia. Neuroscience 27: 175–182
Korf J, Klein HC, Venema K, Postema F (1988) Increases in striatal and hippocampal impedance and extracellular levels of amino acids by cardiac arrest in freely moving rats. J Neurochem 50: 1087–1096
Kvamme E (1983) Glutaminase (PAG). In: Hertz L, Kvamme E, Mcgeer EG, Schousboe A (eds) Glutamine, glutamate, and GABA in the central nervous system. Alan R Liss, New York, pp 51–67
Lehmann A (1987) Alterations in hippocampal extracellular amino acids and purine catabolites during limbic seizures induced by folate injections into the rabbit amygdala. Neuroscience 22: 573–578
Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384: 145–155
Nadler JV, White WF, Vaca KW, Redburn DA, Cotman CW (1977) Characterization of putative amino acid transmitter release from slices of rat dentate gyrus. J Neurochem 29: 279–290
Palaiologos G, Hertz L, Schousboe A (1988) Evidence that aspartate aminotransferase activity and ketodicarboxylate carrier function are essential for biosynthesis of transmitter glutamate. J Neurochem 51: 317–320
Palaiologos G, Hertz L, Schousboe A (1989) Role of aspartate aminotransferase and mitochondrial dicarboxylate transport for release of endogenously and exogenously supplied neurotransmitter in glutamatergic neurons. Neurochem Res 14: 359–366
Passarella S, Atlante A, Barile M, Quagliariello E (1987) Anion transport in rat brain mitochondria: fumarate uptake via the dicarboxylate carrier. Neurochem Res 12: 255–264
Reubi JC (1980) Comparative study of the release of glutamate and GABA, newly synthesized from glutamine, in various regions of the central nervous system. Neuroscience 5: 2145–2150
Sandberg M, Butcher SP, Hagberg H (1986) Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: In vivo dialysis of the rat hippocampus. J Neurochem 47: 178–184
Wakamori M, Ikemoto Y, Akaike N (1991) Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responces in dissociated CNS neurons of the rat. J Neurophysiol 66: 2014–2021
Ward HK, Thanki CM, Bradford HF (1983) Glutamine and glucose as precursors of transmitter amino acids: ex vivo studies. J Neurochem 40: 855–860
Wenthold RJ, Altschuler RA (1983) Immunocytochemistry of aspartate aminotransferase and glutaminase. In: Hertz L, Kvamme E, Mcgeer EG, Schousboe A (eds) Glutamine, glutamate, and GABA in the central nervous system. Alan R Liss, New York, pp 33–50
Yu ACH, Hertz E, Hertz L (1984) Alterations in uptake and release rates for GABA, glutamate and glutamine during biochemical maturation of highly purified cultures of cerebral cortical neurons, a GABAergic preparation. J Neurochem 42: 951–960
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cobo, M., Bruhn, T., Berg, M. et al. Phenylsuccinate reduces KCL-induced release of GABA evidence for the participation of the ketodicarboxylate carrier in the biosynthesis of transmitter-GABA. Amino Acids 5, 377–388 (1993). https://doi.org/10.1007/BF00806956
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806956