Summary
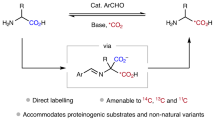
[2-13C]-L-lysine, [3,4-13C2]-L-lysine and [5,6-13C2]-L-lysine are prepared from simple, commercially available, highly enriched starting materials as [2-13C]-glycine, ethyl [1,2-13C2]-bromo acetate, and [1,2-13C2]-acetonitrile. The introduction of the chiral center is based on a general method starting from the bis-lactim ether of cyclo-(D-Val-Gly). The synthesis of (2R)-[5-13C]-3,6-diethoxy-2,5-dihydro-2-isopropylpyrazine is described. The availability of our method for the preparation of specifically enriched bis-lactim ethers allows the synthesis of a great variety of site specific isotopically labelled (L- and D-)α-amino acids. Moreover, intermediate 4-[(2R,5S)-3,6-diethoxy-2,5-dihydro-2-isopropyl-5-pyrazinyl]butyronitrile is a valuable precursor in the synthesis of L-α-aminoadipic acid. The synthetic scheme in this publication makes both L-lysine and L-α-aminoadipic acid13C- or15N-labelled at any position, easily available. The isotopomers of lysine are obtained on a preparative scale in good yields, with 99%13C and high enantiomeric purity (>97% e.e.). Three isotopomers are characterized using various spectroscopic techniques,e.g.,1H NMR,13C NMR and Mass spectrometry.
Similar content being viewed by others
References
van den Berg E, Richardson EE, Lugtenburg J (1987) Convenient syntheses for selectively isotopically labelled acrylonitriles. Synth Comm 17/10: 1189–1198
van den Berg EMM, Baldew AU, de Goede ATJW, Raap J, Lugtenburg J (1988) Synthesis of three isotopomers of L-tryptophan via a combination of organic synthesis and biotechnology. Recl Trav Chim Pays-Bas 107: 73–81
Cappon JJ, Baart J, van der Walle GAM, Raap J, Lugtenburg J (1991) Chemo-enzymatic synthesis of specifically stable-isotope labelled L-glutamic acid and 2-oxoglutaric acid. Recl Trav Chim Pays-Bas 110: 158–166
Cappon JJ, van der Walle GAM, Verdegem PJE, Raap J, Lugtenburg J (1992a) Synthesis of specifically stable-isotope-labeled L-proline via L-glutamic acid. Recl Trav Chim Pays-Bas 111: 517–523
Cappon JJ, Baart J, van der Walle GAM, Verdegem PJE, Raap J, Lugtenburg J (1992b) Chemoenzymatic synthesis of specifically stable-isotope labelled L-glutamic acid, L-glutamine anad L-proline. Indian J Chem Sect B31: 813
Cappon JJ, Witters KD, Verdegem PJE, Hoek AC, Luiten RJH, Raap J, Lugtenburg J (1994) Synthesis of specifically stable-isotope labelled L-histidine. Recl Trav Chim Pays-Bas (accepted for publication)
Gehrke CW, Kuo KC, Kaiser FE, Zumwalt RW (1987) Analysis of amino acids by gas chromatography as theN-trifluoroacetyln-butyl esters. J Assoc Off Anal Chem 70: 160–170
Gelpi E, Koenig WA, Gilbert J, Oró J (1969) Combined gas chromatography-mass spectrometry of amino acid derivatives. J Chromatogr Sci 7: 604–613
Kragl U, Gödde A, Wandrey C, Kinzy W, Cappon JJ, Lugtenburg J (1993) Repetitive batch as an efficient method for preparative scale enzymatic synthesis of 5-azido neuraminic acid and15N-L-glutamic acid. Tetrahedron Asymm 4: 1193–1202
Krapcho AP, Lovey AJ (1973) Decarboxylations of geminal diesters,β-keto esters andα-cyano esters effected by sodium chloride in dimethyl sulfoxide. Tetrahedron Lett 12: 957–961
Lugtenburg J, Mathies RA, Griffin RG, Herzfeld J (1988) Structure and function of rhodopsins from solid state NMR and Resonance Raman spectroscopy of isotopic retinal derivatives. TIBS 15: 388–393
Raap J, Winkel C, de Wit AHM, van Houten AHH, Hoff AJ, Lugtenburg J (1990a) Mass spectrometric determination of isotopically labeled tyrosines and tryptophanes in photosynthetic reaction centers ofRhodobacter sphaeroides R-26. Anal Biochem 191: 9
Raap J, van der Wielen CM, Lugtenburg J (1990b) Enantioselective syntheses of isotopically labelledα-amino acids. Preparation of [ε-13C]-L-aminoadipic acid and five isotopomers of L-lysine with13C,15N and2H in theδ- andε-positions. Recl Trav Chim Pays-Bas 109: 277–286
Raleigh DP, Levitt MH, Griffin RG (1988) Rotational resonance in solid state NMR. Chem Physics Letters 146: 71–104
Roessler F, Hesse M (1977) Das Unterschiedliche massa-spektrometrische Fragmentie-rungsverhalten von Lysinmethylester und dessenN,N′-Diacetylderivat. Helv Chim Acta 60: 380–390
Schöllkopf U (1983) Enantioselective synthesis of nonproteinogenic amino acids. Top Curr Chem 109: 65–83
Smith SO, de Groot H, Gebhard R, Lugtenburg J (1992) Magic angle spinning NMR studies on the metarhodopsin II intermediate of bovine rhodopsin; evidence for an unprotonated Schiff base. Photochem Photobiol 56: 1035–1039
Thompson LK, McDermott AE, Raap J, van der Wielen CM, Lugtenburg J, Herzfeld J, Griffin RG (1992) Rotatational resonance NMR study of the active site structure in bacteriorhodopsin conformation of the Schiffbase linkage. Biochemistry 31: 7931–7938
Williams RM (1989) Synthesis of optically activeα-amino acids. Pergamon Press, Oxford, p 1
Winkel C, Aarts MWMM, van der Heide FR, Buitenhuis EG, Lugtenburg J (1989) Synthesis and NMR-spectroscopy of stable isotope-labelled phenols and L-tyrosines. Recl Trav Chim Pays-Bas 108: 139–146
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raap, J., Wolthuis, W.N.E., Hehenkamp, J.J.J. et al. Enantioselective syntheses of isotopically labelledα-amino acids Preparation of specifically13C-labelled L-lysines. Amino Acids 8, 171–186 (1995). https://doi.org/10.1007/BF00806490
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00806490