Summary
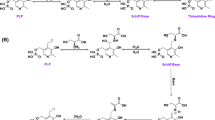
S-(2-oxo-2-carboxyethyl)homocysteine (OCEHC), produced by the enzymatic monodeamination of cystathionine, is known to cyclize producing the seven membered ring of cystathionine ketimine (CK) which has been recognized as a cystathionine metabolite in mammals. Studies have been undertaken in order to find the best conditions of cyclization of synthetic OCEHC to CK and for the preparation of solid CK salt product. It has been found that ring closure takes place at alkaline pH and is highly accelerated in 0.5 M phosphate buffer. The sodium salt of CK has been prepared by controlled additions of NaOH to water-ethanol solution of OCEHC under N2 atmosphere. A solid product is obtained which, dissolved in water, shows the spectral features of CK. Solutions of the sodium salt of CK show the presence of a pH depending reversible equilibrium with the open OCEHC form. Plot of the absorbance at 296 nm in function of pH indicates that at pH 9 the compound is completely cyclized while at pH 6 is totally in the open OCEHC form. At intermediate pHs variable ratios between the two forms occur. According to the results obtained by the spectral analysis, HPLC assays of the sodium salt of CK show different patterns depending on the pH of the elution buffer.
Similar content being viewed by others
Abbreviations
- CK:
-
cystathionine ketimine
- OCEHC:
-
S-(2-oxo-2-carboxyethyl) homocysteine
- HPLC:
-
high performance liquid chromatography
References
Antonucci A, Pecci L, Fontana M, Cavallini D (1990) Influence of diet on cystathionine ketimine and lanthionine ketimine content in human urine. Ital J Biochem 39: 100–105
Cavallini D, Costa M, Pensa B, Coccia R (1985a) The conversion of L-cystathionine into the cyclic ketimine form by heated rat liver extracts containing cystathionase and transaminase activities. Biochem Int 10: 641–646
Cavallini D, Pecci L, Matarese RM, Ricci G, Achilli M (1985b) Gas-chromatographic mass-spectrometric detection of 1,4-hexahydrothiazepine-3,5-dicarboxylic acid (cyclothionine) in bovine brain. J Biol Chem 260: 15577–15579
Costa M, Pensa B, Cavallini D (1984) Ketimine formation by interacting L-cystathionine with glyoxylic acid. IRCS Med Sci 12: 468–469
Costa M, Pensa B, Blarzino C, Cavallini D (1985) New enzymatic changes of L-cystathionine catalyzed by bovine tissue extracts. Physiol Chem Phys 17: 107–111
Costa M, Pensa B, Fontana M, Foppoli C, Cavallini D (1986) Transamination of L-cystathionine and related compounds by a bovine liver enzyme. Possible identification with glutamine transaminase. Biochim Biophys Acta 881: 314–320
Matarese RM, Pecci L, Ricci G, Cavallini D (1984) Gas-chromatographic determination of thiazine and thiazepine derivatives of biological interest. J Chromatogr 294: 413–418
Matarese RM, Pecci L, Ricci G, Nardini M, Antonucci A, Cavallini D (1987) Hexahydro-1,4-thiazepine-3,5-dicarboxylic acid and thiomorpholine-3,5-dicarboxylic acid are present in normal human urine. Proc Natl Acad Sci USA 84: 5111–5114
Nardini M, Kodama H, Ricci G, Federici G, Cavallini D (1985) Enzymatic production of S-(2-hydroxy-2-carboxyethyl)homocysteine. Biochem Int 11: 789–794
Pecci L, Antonucci A, Nardini M, Cavallini D (1988a) Detection of cystathionine and lanthionine ketimines in human urine. Biochem Int 17: 877–883
Pecci L, Costa M, Pinnen F, Antonucci A, Cavallini D (1988b) Properties of the phenylthiohydantoine derivatives of some sulfur containing cyclic amino acids. Physiol Chem Phys Med NMR 20: 199–203
Ricci G, Federici G, Lucente G, Achilli M, Cavallini D (1982) L-lanthionine oxidation by snake venom L-amino acid oxidase. Physiol Chem Phys 14: 193–199
Ricci G, Santoro L, Achilli M, Matarese RM, Nardini M, Cavallini D (1983) Similarity of the oxidation products of L-cystathionine by L-amino acid oxidase to those excreted by cystathioninuric patients. J Biol Chem 258: 10511–10517
Ricci G, Vesci L, Matarese RM, Antonucci A, Maggio A, Pecci L, Cavallini D (1990) Detection of cystathionine ketimine in bovine cerebellum. J Neurochem 55: 1599–1602
Watanabe H, Fujita Y, Sugahara K, Kodama H, Ohmori S (1991) Identification of Nac-HCPC and Nac-β-CEC, and qualitative analyses of sulfur amino acids in the urine of a patient with cystathioninuria using liquid chromatography/atmospheric pressure ionization mass spectrometry. Biol Mass Spectr 20: 602–608
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Solinas, S.P., Pecci, L., Montefoschi, G. et al. Reversible cyclization ofS-(2-oxo-2-carboxyethyl)-L-homocysteine to cystathionine ketimine. Amino Acids 4, 133–140 (1993). https://doi.org/10.1007/BF00805809
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00805809