Summary
It is well known that increased cross linking of proteins due to nonenzymatic glycosylation occurs in diabetic animals and humans leading to accumulation of proteins (e.g. collagen). This in turn is strongly associated with diabetic long term complications.
We developed a noninvasive method for studying in vivo cross linking and its pharmacological inhibition by L-arginine in a blind placebo controlled study with crossing over of two treatment periods of three months each.
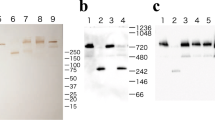
Glycemic control was assessed by determining blood glucose, HbA1c, fructosamine, and total glycosylated hemoglobin. The patients were randomly assigned to two treatment groups A (n = 14) and B (n = 16). 20 healthy volunteers served as controls. Treatment consisted of two daily dosages of 1 g L-arginine free base. Cross linking of a human serum protein (IgG) was assessed by SDS polyacrylamide gel electrophoresis and subsequent Western blotting.
Diabetic patients showed a statistically increased number of cross links compared to normal controls (Group A: 3.6 vs 2.0 bands, group B: 3.8 vs 2.0 bands). L-arginine led to a significant reduction of cross links in both treatment groups (Group A: 3.6 to 2.1 bands, group B: 3.8 to 2.5 bands).
The described noninvasive method for assessing in vivo cross linking requires onlyµl amounts of serum and could serve to monitor protein cross linking in patients with diabetes mellitus.
Similar content being viewed by others
References
Barbul A, Fichell RS, Shimadzu S, et al (1985) Intravenous hyperalimentation with high arginine levels improved wound healing and immune functions. J Surg Res 38: 328–334
Brownlee M, Vlassara H, Kooney T, Ulrich P, Cerami A (1986) Aminoguanidine prevents diabetes induced arterial wall protein cross linking. Science 232: 1629–1632
Igaki N, Sakai M, Hata H, Oimomi M, Baba S, Kato H (1990) Effects of 3-deoxyglucosone on the Maillard reaction. Clin Chem 36: 631–634
Jones AF, Jennings PE, Wakefield A, Winkles J, Lunec J, Barnett AH (1988) The fluorescence of serum proteins in diabetic patients with and without retinopathy. Diab Med 5: 547–551
Kato H, Cho RK, Okitani A, Hayase F (1987) Responsibility of 3-deoxyglucosone for the glucose induced polymerization of proteins. Agric Biol Chem 51: 683–689
Lämmli UK (1970) A cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–682
Lubec G, Bartosch B, Mallinger R, et al. (1990a) The effect of substance L on glucose mediated cross links of collagen in thedb/db mouse. Nephron 86: 678–694
Lubec G, Bartosch B, Mallinger R, et al. (1990b) The effect of substance L on glucose mediated cross links of collagen in thekk-mouse. In: Lubec G, Rosenthal GA (eds) Amino acids. Escom, Leiden, pp 670–675
Lubec G, Pollak A (1980) Reduced susceptibility of nonenzymatically glycosylated glomerular basement membranes to proteases. Renal Physiol; 3: 4–9
Lunec J (1983) Free radical mediated aggregation of IgG. Agents Actions 15: 37–38
Lubec G, Vierhapper H, Bailey AJ, Damjancic P, Fasching P, Sims TJ, Kampel D, Popow C, Bartosch B (1991) Influence of L-arginine on glucose mediated collagen cross links precursors in patients with diabetes mellitus. Amino Acids 1: 35–41
Monnier VM, Kohn R, Cerami A (1984) Accelerated age related browning of human collagen in diabetes mellitus. Proc NAS (USA), 81: 583–587
Monnier VM, Vishwanath V, Frank KE, et al (1986) Relation between complications of type I diabetes mellitus and collagen linked fluorescence. N Engl J Med 314: 402–408
Oimomi M, Hata F, Igaki N, Nakamichi T, Baba S, Kato H (1989) Purification of alphaketoaldehyde dehydrogenase from human liver and its possible significance in the control of glycation. Experientia 45: 463–466
SAS User's guide: Statistics (1985) Version 5 th edn. SAS Institute, Cary, NC
Schnider GL, Kohn RR (1981) Effects of age and diabetes mellitus on the solubility and nonenzymatic glycosylation of human skin collagen. J Clin Invest 67: 1630–1635
Vlassara H, Brownlee M, Cerami A (1984) Accumulation of diabetic rat peripheral nerve myelin by macrophages increases with the presence of advanced glycosylation end products. J Exp Med 160: 197–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lubec, B., Weninger, M., Popow, C. et al. In vivo monitoring of serum protein cross linking in patients with diabetes mellitus. Evidence for pharmacological modification of immunoglobulin G cross links. Amino Acids 4, 111–119 (1993). https://doi.org/10.1007/BF00805806
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00805806