Abstract
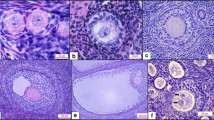
“In vitro” studies, commonly using porcine ovarian follicles, may generate inconsistent results when atretic follicles are not eliminated from the pool of experimental follicles. The present experiment was conducted to test the practical value of the macroscopie identification of large porcine follicles, which were assumed to be atretic. Histological observations of hematoxylin-eosin stained follicular sections confirmed the results of the macroscopic classification. The follicles classified as presumably abnormal revealed signs of atresia at the light microscopic level. Such follicles (type 2) showed decreased levels of estradiol and androgens in comparison with the healthy-looking follicles (type 1).
Steroid analysis also revaled that practically all estradiol from an ovarian follicle could be detected in the follicular fluid, whereas androgens extracted from follicular fluid represented approximately half of the total amount of follicular androgens.
The experimental results indicate that the introduced macroscopic classification could be helpful in eliminating follicles with an impaired steroid function.
Similar content being viewed by others
References
Ainsworth L, Tsang BK, Downey BR, Marcus GJ (1990) The synthesis and actions of steroids and prostaglandins during follicular maturation in pig. J Reprod. Fertil. [Suppl] 40: 137–150.
Akins EL, Morrisette MC (1969) Gross ovarian changes during estrus cycle of swine. Amer. J. Res. 29: 1953–1955.
Carson RS, Findlay JK, Clarke IJ, Burger HG (1981) Estradiol, testosterone and androstenedione in ovine follicular fluid during growth and atresia of ovarian follicles. Biol. Reprod. 24: 105–113.
Channing CP, Ledwitz-Righby F (1975) Methods for assessing hormone mediated differentiation of ovarian cells in culture and short term incubations. In: Methods in Enzymology XXXIX Part D/J., B. Hardman and B. O'Maddley. Academic Press, New York, London: 183–230.
Dufau ML, Catt KJ, Tsuruhara T, Ryan D (1972) Radioimmunoassay of plasma testosterone. Clin. Chim. Acta 37: 109–116.
Farookhi R (1981) Atresia: A Hypothesis. In: Dynamics of Ovarian Function. Schwartz NB, Hunzicker-Dunn M. (eds.). Raven Press, New York: 13–25.
Greenwald GS, Terranova PF (1988) Follicular selection and its control. In: The Physiology of Reproduction, Knobil E, and Neill J, (eds.) Raven Press, New York: 387–445.
Hubbard ChJ, Oxberry B (1991) Follicular atresia. In: Ultrastructure of the Ovary. Familiari G, Makaba S, Motto PM, (eds.). Kluwer Academic Publishers: 273–285.
Hotchkiss J, Atkiinson LE, Knobil E (1971) Time course of serum estrogen and luteinizing hormone (LH) concentrations during menstrual cycle of rhesus monkey. Endocrinology 89: 177–189.
Hunter MG, Wiesak T (1990) Evidence for and implications of follicular heterogeneity in pigs. J. Reprod. Fertil. 40 [Suppl]: 163–177.
Maxson WAS, Haney AF, Schomberg DW (1985) Steroidogenesis in porcine atretic follicle: loss of aromatase activity in isolated granulosa and theca. Biol. Reprod 33: 495–501.
Rátky J, Brüssow KP (1992) Ovarian morphological changes during the estrus cycle in gilts. In: Proceedings of The 12th International Congres on Animal Reproduction. The Hague, The Netherlands. Vol. 1: 102–104.
Ryan JR (1981) Follicular atresia: some speculations of biochemical markers and mechanisms. In: Dynamics of Ovarian Function. Schwartz NB, Hunzicker-Dunn M (eds.). Raven Press New York: 1–11.
Stoklosowa S, Gregoraszczuk E, Channing CP (1982) Estrogen and progesterone secretion by isolated cultured porcine thecal and granulosa cells. Biol. Repord. 26: 943–952.
Tsafriri A, Braw RH (1984) Experimental approaches to atresia in mammals. Oxf. Rev. Repord. Biol. 6: 226–265.
Uilenbroek JThJ, Van der Schoot P, Woutersen PJA (1981) Changes in steroidogenic activity of preovulatory rat follicles after block-age of ovulation with nembutal. In: Dynamics of Ovarian Function. Schwartz N.B, Hunzicker-Dunn M. (Eds.). Raven Press, New York: 41–61.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dudek, D. Can macroscopic identification of large ovarian follicles be useful in eliminating follicles with impaired steroid function?. Cytotechnology 14, 147–153 (1994). https://doi.org/10.1007/BF00758179
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00758179