Abstract
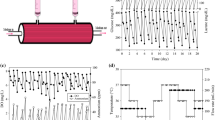
The growth yields for glucose and glutamine of murine hybridoma cells entrapped in collagen gel particles were examined during the growth phase. The immobilized hybridoma cells were cultivated in a fluidized bed fermenter where the medium was circulating to supply oxygen separately. Procedures to supply an alkaline solution for adjusting the pH level strongly affected the growth yields. A direct supply of the alkaline solution to the cultivation system reduced both the growth yields for glucose and glutamine, probably due to a local increase in pH level. On the other hand, when fresh medium in which the pH was adjusted to around 8.5 was added to the cultivation system, the growth yields were unchanged even at the same pH level as when direct alkaline supply was used. These results suggest that an indirect alkaline supply could be recommended to ajust the pH level when using medium-circulating-fermenters.
Similar content being viewed by others
References
Bantemeyer H, Wallerius C and Lehmann (1992) Optimal medium use for continuous high density perfusion processes. Cytotechnology 9: 59–67.
Familletti PC and Fredericks JE (1988) Techniques for mammalian cell immobilization. Bio/Technol. 6: 41–44.
Feder J and Tolbert WR (1983) The large scale cultivation of mammalian cells. Scientific American 248: 24–31.
Glacken MW, Fleischaker RJ and Sinskey AJ (1983) Large scale production of mammalian cells and their products: engineering principles and barriers to scale-up. Annal. NY. Acad. Sci. 423: 243–249.
Goegen JL, Marc A and Engasser JM (1992) Comparison of specific rates of hybridoma growth and metabolism in batch and continuous cultures. Cytotechnology 10: 147–155.
Griffiths JB, Looby D and Racher AJ (1992) Maximization of perfusion systems and process comparison with batch-type cultures. Cytotechnology 9: 3–9.
Hamamoto K, Ishimaru K and Tokashiki M (1989) Perfusion culture of hybridoma cells using a centrifuge to separate cells from culture mixture. J. Ferment. Bioeng. 67: 190–194.
Knight P (1989) Chromatography: 1989 report. Bio/Technol. 7: 1–11.
Luan YT, Mutharasan R and Magee WE (1987) Factors governing lactic acid formation in long term cultivation of hybridoma cells. Biotechnol. Letter 9: 751–756.
Lydersen BK, Pugh GG, Paris MS, Sharma BP and Noll LA (1985) Ceramic matrix for large scale animal cell culture. Bio/Technology 3: 63–67.
Mathiot B, Perani A, Dumas D, Maugras M, Didelon J and Stoltz JF (1993) Increase of hybridoma productivity using an original dialysis culture. Cytotechnnology 11: 41–48.
Murakami H, Masui H, Sato GH, Sueoka N, Chow TP and Sueoka TK (1982) Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc. Natl. Acad. Sci. USA 79: 1158–1162.
Nilsson K (1987) Methods for immobilizing animal cells. TIBTECH 5: 73–78.
Olsson L and Mathe G (1982) Emerging immunological approaches to treatment of neoplastic diseases. In: Mathe G, Bonadonna G and Salmon S (eds) Recent results in cancer research. (vol. 80. p334–337). Springer-Verlag.
Phillips HA, Scharer JM, Bols NC and Moo-Young M (1992) Design and performance of a trickle-bed bioreactor with immobilized hybridoma cells. Cytotechnology 9: 29–40.
Ramirez OT and Mutharasan R (1989) Physical immobilization characteristic of a hybridoma in a glass bead packed-bed reactor. Biotechnol. Bioeng. 33: 1072–1976.
Ritz J and Schlossman SF (1982) Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood: 591–11.
Sato S, Kawamura K and Fujiyoshi N (1983) Animal cell cultivation for production of biological substances with a novel perfusion culture apparatus. Tissue Culture Met. 8: 167–171.
Shirai Y, Hashimoto K and Takamatsu H (1992) Growth kinetics of hybridoma cells in high density culture 73: 159–165.
Shirai Y, Hashimoto K, Yamaji H and Tokashiki M (1987) Continuous production of monoclonal antibody with immobilized hybridoma cells in an expanded bed fermentor. Appl. Microb. Biotechnol. 26: 495–499.
Van Brunt J (1986) Immobilization mammalian cells: The gentle way to productivity. Bio/Technology 4: 505–510.
Vornakis JN and Runstadler PW (1989) Microenvironment: The key to improved cell culture products. Bio/Technil. 7: 153–145.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shirai, Y., Yamaguchi, M., Kobayashi, A. et al. Change in growth kinetics of hybridoma cells entrapped in collagen gel affected by alkaline supply. Cytotechnology 14, 129–146 (1994). https://doi.org/10.1007/BF00758178
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00758178