Abstract
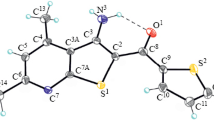
Reaction of 2,4,5-trioxo-7-aminopyrido[2,3-d]pyrimidines with acylating agents takes place both at the amino group and at the cyclic nitrogen atom. Reaction of these compounds with formic acid, chloroacetyl chloride in pyridine, cyanoacetic acid in the presence of acetic anhydride, and oxalyl chloride leads to monoacylation at the amino group but with methyl chloroformate it is the product of acylation at the cyclic nitrogen. Refluxing in acetic anhydride gave mono-, di-, and triacetyl derivatives. The structures of these compounds were proved using spectral data.
Similar content being viewed by others
Literature Cited
N. M. Smirnova, N. M. Cherdantseva, V. M. Nesterov, and T. S. Safonova, USSR Author's Certificate No. 716,279;Byul. Izobr., No. 36, 260 (1987).
W. Pfleiderer and E. Ziedick,Annalen 612, 163 (1958).
W. Pfleiderer and J. Strauss,Annalen 612, 173 (1958).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 811–814, June, 1990.
Rights and permissions
About this article
Cite this article
Burova, O.A., Smirnova, N.M. & Safonova, T.S. Pyrido[2,3-d]pyrimidines. 1. Acylation of 2,4,5-trioxo-7-amino-8H-pyrido [2,3-d]pyrimidines. Chem Heterocycl Compd 26, 677–680 (1990). https://doi.org/10.1007/BF00756423
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00756423