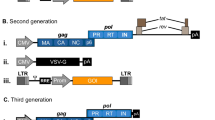
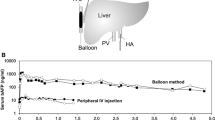
Abstract
Viral vectors provide a highly efficient method for the transfer of foreign genes into a variety of quiescent or dividing eukaryotic cells from many animal origins. While recombinant vectors derived from an increasing number of mammalian viruses (herpes simplex virus, autonomous and non-autonomous parvoviruses, poxviruses, retroviruses, adenoviruses available today, vectors based on murine retroviruses and human adenoviruses constitute preferential candidates for the delivery of marker or therapeutic genes into human somatic cells. The availability of such vectors has made possible the recent transition of human gene therapy from laboratory benches to clinical settings. Most current recombinant vectors have been generated by deleting essential viral genes in order to make space available for the introduction of passenger genes. Such vectors are therefore unable to replicate in the absence of these critical gene products and their production relies on the development of stable complementation cell lines providingin trans the missing viral functions. Although complementation (or packaging) cell lines are available for both adenovirus and retrovirus vectors, their respective drawbacks still limit their use to research applications and phase I clinical trials. The future success or failure of human gene therapy will therefore rely on the production of improved generations of packaging cell lines that can produce safer and more efficient vectors which are fully adapted to large scale production and clinical applications.
Similar content being viewed by others
References
Ali M, Lemoine NR and Ring CJA (1994) The use of DNA viruses as vectors for gene therapy. Gene Ther 1: 367–384.
Anderson WF (1992) Human gene therapy. Science 256: 808–813.
Babiss LE, Young CSH, Fisher PB and Ginsberg HS (1983) Expression of adenovirus E1A and E1B gene products and theEscherichia coli XGPRT gene in KB cells. J Virol 46: 454–465.
Bartholomew RM, Esser AF and Muller-Eberhard HJ (1978) Lysis of oncornaviruses by human serum: isolation of the viral complement (C1) receptor and identification as p 15E. J Exp Med 147: 844–853.
Bender MA, Palmer TD, Gelinas RE and Miller AD (1987) Evidence that the packaging signal of Moloney leukemia virus extends into thegag region. J Virol 61: 1639–1646.
Bett AJ, Haddara W, Prevec L and Graham FL (1994) An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA 91: 8802–8806. Carroll R, Lin JT, Dacquel EJ, Mosca JD, Burke DS and StLouis DC (1994) A human immunodeficiency virus type-HIV-1)-based retroviral vector system utilizing stable HIV1 packaging cell lines J Virol 68: 6047–6051. Coffin, JM (1990) Retroviridae and their replication. In: Fields BN, Knipe DM (eds.) Virology, 2nd ed. New-York, NY: Raven Press, Ltd, pp 1437–1489.
Crystal RG, McElvaney NG, Rosenfeld MA, Chu CS, Mastrangeli A, Hay JG, Brody SL, Jaffe HA, Heissa NT and Daniel C (1994) Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nature Genet 8: 42–51.
Danos O and Mulligan R (1988) Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ransges. Proc Natl Acad Sci USA 85: 6460–6464.
Deminie CA and Emerman M (1994) Functional exchange of an oncoretrovirus and a lentivirus matrix protein. J Virol 68: 4442–4449.
Donahue RE, Kessler SWW, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo KM and Nienhuis AW (1992) Helper virus induced T cell lymphomas in non-human primates after retroviral mediated gene transfer. I Exp Med 176: 1124–1135.
Gallimore PH, Grand RJA and Byrd PJ (1986) Transformation of human embryo retinoblasts with simian virus 40, adenovirus and ras oncogenes. Anticancer Res 6: 499–508.
Gulizia J, Dempsey MP, Sharova N, Bukrinsky MI, Spitz L, Goldfarb D and Stevenson M (1994) Reduced nuclear import of HIV-1 preintegration complexes in the presence of a prototypic nuclear targeting signal. J Virol 68: 2021–2015.
Graham FL (1984) Transformation by and oncogenicity of human adenoviruses. In Ginsberg HS (ed) The Adenovirus, New-York Plenum Press. pp 339–398.
Graham FL and Prevec L (1992) Adenovirus based expression vectors and recombinant vaccines. In: Ellis RW, (ed) New Approaches to Immunological Problems Butterworth-Heinemann, pp 363–390.
Graham FL, Smiley J, Russel WC and Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36: 59–72.
Grodzicker T and Klessig DF (1980) Expression of unselected adenovirus genes in human cells co-transformed with the HCV-1 TK gene and adenovirus 2 DNA. Cell 21: 453–463.
Haddada H, Ragot T, Cordier L, Duffour MT and Perricaudet M (1993) Adenoviral interleukin 2 gene transfer into P815 tumor cells abrogates tumorigenicity and induces antitumoral immunity in mice. Hum Gene Ther 4: 702–711.
Halbert DN, Cutt JR and Shenk T (1985) Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host protein cell shutoff. J Virol 56: 250–257.
Hantzopoulos PA Sullenger BA, Ungers G and Gilboa E (1989) Improved gene expression upon transfer of the adenosine deaminase minigene outside the transcriptional unit of a retroviral vector. Proc Natl Acad Sci USA 86: 3519–3523.
Hitt MM and Graham F (1990) Adenovirus E1A under the control of heterologous promoters: wide variation in E1A expression levels has little effects on virus replication. Virology 179: 667–678.
Horwitz MS (1990) Adenoviridae and their replication. in: Fields NB, Knipe DMet al. Virology. Raven Press Ltd pp 1679–1721.
Hwang LHS and Gilboa E (1984) Expression of genes introduced into cells by retroviral infection is more efficient than that of genes introduced into cells by DNA transfection. J Virol 50: 417–424.
Imler JL, Chartier C, Dreyer D, Dieterle A, Sainte-Marie M, Faure T, Pavirani A and Mehtali M (1995a) Novel complementation cell lines derived from human lung carcinoma A549 cells support the growth of E1-deleted adenovirus vectors. Gene Ther. In Press.
Imler JL, Dieterle A, Dreyer D, Mehtali M and Pavirani A (1995b) An efficient procedure to select and recover recombinant adenovirus vectors. Gene Ther 2: 263–268.
Jolly D (1994) Viral vectors for gene therapy. Cancer Gene Ther 1: 51–64.
Ketner G, Spencer F, Tugendreich S, Connelly C and Hieter P (1994) Efficient manipulation of the human adenovirus genome as an infectious yeast artificial chromosome clone. Proc Natl Acad Sci USA 91: 6186–6190.
Kotani H, Newton PB, Zhang S, Chiang YL, Otto E, Weaver L, Blaese MR, Anderson, WF and McGarrity GJ (1994) Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther 5: 19–28.
Lewis P, Hensel M and Emerman M (1992) HIV-1 infection of cells arrested in the cell cycle. EMBO J 11: 3053–3058.
Lochmuller H, Jani A, Huard Jet al. 61994) Emergence of early region-1 containing replication competent adenovirus in stocks of replication defictive adenovirus recombinants (ΔE1±ΔE3) during multiple passages in 293 cells. Hum Gen Ther 5: 1485–1493.
Mann R, Mulligan R and Baltimore D (1983) Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell 33: 153–159.
Markowitz D, Golff S and Bank A (1988) Construction and use of a safe and efficient amphotropic packaging cell line. Virol 167: 400–406.
Miller, AD (1990) Retrovirus packaging cell lines. Hum gene Ther 1: 5–14.
Miller AD (1992) Human gene therapy comes of age. Nature 357: 455–460.
Miller AD, Buttimore C (1986) Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production Moll Cell Biol. 6: 2895–2901.
Miller AD, Miller DG, Garcia JV and Lynch CM (1993) Use of retroviral vectors for gene transfer and expression. Methods in Enzymol 217:581–599.
Miller AD and Rosman GJ (1989) Improved retroviral vectors for gene transfer and expression. Bio Tech 7:980–990.
Miller DG, Edwards RH and Miller AD (1994) Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA 91:78–82.
Morgenstem JP and Land H (1990) Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper free packaging cell line. Nucl Acids Res 18:3587–3596.
Mulligan, RC (1993) The basic science of gene therapy. Science 260:926–932.
Otto E, Jones-Trower A, Vanin EF, Stambaugh K, Mueller SN, Anderson WF and McGarrity GJ (1994) Characterization of a replication-competent retrovirus resulting from a recombination of packaging and vector sequences. Hum Gen Ther 5:567–575.
Overall RW, Weisser KE and Cosman D (1988) Stably transmitted triple promoter retroviral vectors and their use in transformation of primary mammalian cells. Mol Cell Biol 8: 1803–1808.
Paul RW, Morris D, Hess BW, Dunn J and Overall RW (1993) Increased viral titer through concentration of viral harvests from retroviral packaging lines. Hum Geno Ther 4:609–615.
Rao L, Debbas M, Sabbatini P, Hockebery D, Korsmeyer S and White E (1992) The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19kD and Bcl-2 proteins. Proc Natl Acad Sci USA 89:7742–7746.
Rosenfeld MA, Yoshimura K, Trapnell BC, Yoneyama K, Rosenthal EF, Dalemeans W, Fukayama M, Bargon J, Stier LE, Stratford-Perricaudet L, Perricaudet M, Guggino WB, Pavirani A, Lococq JP and Crustal RG (1992)In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway eepithelium. Cell 68:143–155.
Shiroki K, Saito I, Maruyama K, Fukui Y, Imatani Y, Oda KI and Shimojo H (1983) Expression of adenovirus type 12 early region 1 in KB cells transformed by recombinants containing the gene. J. Virol 45:1074–1082.
Takeuchi Y, Cosset FL, Lachmann PJ, Okada H, Weiss RA and Collins MK (1994) Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol 68:8001–8007.
Vanin EF, Kaloss M, broscius C and Nienhuis AW (1994) Characterization of replication competent retroviruses from non-human primates with virus-induced T cell lymphomas and observations regarding the mechanism of oncogenesis. J Virol 68:4241–4250.
White E and Capriani R (1989) Specific disruption of intermediate filaments and the nuclear lamina by the 19-KDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci USA 86:9886–9890.
White E, Sabbatini P, Debbas M, Wold WSM, Kusher DI and Gooding L (1992) The 19 kD adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor factor α. Mol Cell Biol 12:2570–2580.
Wickham TJ, Mathias P, Cheresh DA and Nemerow GR (1993) Integrins αbβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309–319.
Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinbert RA and Harlow E (1988) Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature 334:124–129.
Yang Y, Ertl HCJ and Wilson J (1994) MHC classI-restricted cytotoxic Thymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1: 433–422.
Yang Y, Nunes FA, Berencsi K, Furth EE, Conczol E and Wilson JM (1994) Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA 91:4407–4411.
Yu SF, Von Rüden T, Kantoff PW, Garber C, Seiberg M, Rüther U, Anderson WF, Wagner EF and Gilboa E (1986) Self-inactivating retroviral vectors designated for transfer of wholle genes into mammalian cells. proc Natl Acad Sci USA 83:3194–3198.
Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE and Welsh MJ (1993) Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell 75:207–216.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mehtali, M. Complementation cell lines for viral vectors to be used in gene therapy. Cytotechnology 19, 43–54 (1995). https://doi.org/10.1007/BF00749754
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00749754