Abstract
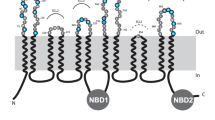
The MDR1-P-glycoprotein binding sites of three different murine monoclonal antibodies (MM4.17, MM6.15 and MC57), directed towards living, intact human multidrug-resistant cells were investigated in order to study P-glycoprotein topology. By using synthetic peptide scanning, we demonstrated that well-defined regions localized on the predicted first, fourth and sixth extracellular loops are external. On the basis of the structure of MM6.15 epitope, which is distributed on the above three different extracellular loops (and thus is discontinuous), P-glycoprotein molecules result to be differently organized in the lipid bilayer. Moreover, the outcome of the MC57 and MM4.17 epitopes localization experiments, obtained through the use of phage-displayed peptide libraries, represent an additional challenge to the classical 12-transmembrane domain model of P-glycoprotein, since they agree with the novel topography of the molecule (10-transmembrane domain), which was recently proposed on the basis of biochemical and expression studies.
Similar content being viewed by others
References
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM and Robinson IB (1986) Internal duplication and homology with bacterial transport protein in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47: 381–389.
Cenciarelli C, Currier SJ, Willingham MC, Thiebaut F, Germann U, Rutherford AV, Gottesman MM, Barca S, Tombesi M, Morrone S, Santoni A, Mariani M, Ramoni C, Dupuis ML and Cianfriglia M (1991) Characterization by somatic cell genetics of a monoclonal antibody to the MDR1 gene product (P-glycoprotein): Deterimnation of P-glycoprotein expression in multi-drug-resistant KB and CEM cell variants. Int J Cancer 47: 533–543.
Cianfriglia M, Romagnoli G, Tombesi M, Poloni F, Falasca G, Di Modugno F, Castagna M and Chersi A (1995) P-glycoprotein epitope mapping. II. The murine monoclonal antibody MM6. 15 to human multidrug-resistant cells binds with three distinct loops in the MDR1-P-glycoprotein extracellular domain. Int J Cancer 61: 142–147.
Cianfriglia M, Willingham MC, Tombesi M, Scagliotti GV, Frasca G and Chersi A (1994) P-glycoprotein epitope mapping. I. Identification of a linear human-specific epitope in the fourth loop of the P-glycoprotein extracellular domain by MM4. 17 murine monoclonal antibody to human multidrug-resistant cells. Int J Cancer 56: 153–160.
Eisenberg D, Schwarz E, Komaromy M and Wall R (1984) Analysis of membrane and surface proteins sequences with a hydrophobic moment plot. J Mol Biol 179: 125–142.
Felici F, Luzzago A, Monaci P, Nicosia A, Sollazzo M and Traboni C (1995) Peptide and protein display on the surface of filamentous bacteriophage. In: El-Gewely R (ed.) Biotecnology Ann Rev Vol. 1, Ch. 5 Elsevier Science BV, Amsterdam.
Georges E, Tsumo T and Ling V (1993) Topology of P-glycoprotein as determined by epitope mapping of MRK-16 antibody. J Biol Chem 268: 1792–1798.
Germann UA, Pastan I and Gottesman MM (1993) P-glycoproteins: mediators of multidrug resistance. Semin Cell Biol 4: 63–76.
Gottesman M and Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62: 385–427.
Jachez B, Cianfriglia M and Loor F (1994) Modulation of human P-glycoprotein epitope expression by temperature and/or resistance-modulating agents. Anti-Cancer Drugs 5: 655–665.
Poloni F, Palumbo S, Cianfriglia M and Felici F (in press) selection of phage-displayed peptides mimicking an extracellular epitope of human MDR1-P-glycoprotein. Physioll. Chem. Phys. Med. NMR.
Poloni F, Romagnoli G, Cianfriglia M and Felici F (1995) Isolation of antigenic mimics of MDR1-P-Glycoprotein by phage-displayed peptide libraries. Int J Cancer 61: 727–731.
Skach WR, Calayag MC and Lingappa VR (1993) Evidence for an alternate model of human P-glycoprotein structure and biogenesis. J Biol Chem. 268: 6903–6908.
Ueda K, Cardarelli C, Gottesman MM and Pastan I (1987) Expression of a full-length cDNA for the human MDR1 (P-glycoprotein) gene confers multi-drug-resistance to colchicin, doxorubicin, and vinblastine. Proc Natl Acad Sci (USA) 84: 3004–3008.
Zhang JT and Ling V (1991). Study of membrane orientation and glycosylated extracellular loops of mouse P-glycoprotein byin vitro translation. J Biol Chem 266: 18224–18232.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cianfriglia, M., Poloni, F., Signoretti, C. et al. Topology of MDR1-P-glycoprotein as indicated by epitope mapping of monoclonal antibodies to human MDR cells. Cytotechnology 19, 247–251 (1996). https://doi.org/10.1007/BF00744220
Issue Date:
DOI: https://doi.org/10.1007/BF00744220