Conclusions
1. The resin is a convenient source of the isolation of the individual gibberellins and other acidic and neutral metabolites.
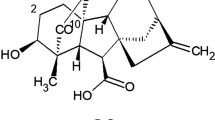
2. By means of partition chromatography in a column of KSK silica gel treated with phosphate buffer having pH 6.4, it has been possible to separate gibberellins A4 and A7 and an isomer of gibberellin A7-the hydroxylactone acid (II).
3. Gibberellin A13 has been isolated from the resin remaining after the evaporation of the mother solutions from the crystallization of industrial gibberellin, and the structure of the isomeric trimethyl esters obtained from it has been elucidated.
4. For the PG-7 strain, the connection observed previously for the F-6 strain between the relative stimulation of the biosynthesis of the gibberellins and kaurene on the one hand, and the relative suppression of the biosynthesis of highly oxidized diterpenes (kaurenolides and fujenal) on the other hand, has been confirmed qualitatively.
Similar content being viewed by others
References
V. F. Kucherov, I. A. Gurvich, A. V. Simolin, and I. M. Mil'shtein, DAN SSSR,163, 765, 1965.
G. S. Muromtsev and L. A. Pen'kov, The Gibberellins [in Russian], Moscow, pp. 107–108, 1962.
B. E. Cross, H. B. Galt, and I. R. Hanson, Tetrah.,18, 451, 1962.
P. C. Aldridge, I. R. Hanson, and T. P. C. Mulholland, J. Chem. Soc., 3539, 1965.
E. P. Serebryakov, V. F. Kucherov, and G. S. Muromtsev, KhPS [Chemistry of Natural Compounds],2, 55, 1966.
H. B. Galt, J. Chem. Soc., 3134, 1965.
R. A. Bell, R. E. Ireland, and L. P. Mauder, J. Org. Chem.,31, 2536, 1966.
J. Fusca, I. Kuhr, M. Podojil, and V. Šervick, Folia microbiol.,6, 1, 1961.
E. P. Serebryakov, A. V. Simolin, V. F. Kucherov, G. S. Muromtsev, and L. P. Dubovaya, KhPS [Chemistry of Natural Compounds], no. 5, [in this issue], p. 156, 1969.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 5, No. 3, pp. 163–169, 1969
Rights and permissions
About this article
Cite this article
Simolin, A.V., Serebryakov, E.P., Kucherov, V.F. et al. Gibberellins and substances related to them. Chem Nat Compd 5, 139–143 (1969). https://doi.org/10.1007/BF00636005
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00636005