Abstract
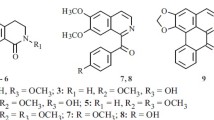
The roots of theHaplophyllum obtusifolium Ledeb. have yielded robustine, dictamnine, skimmianine, γ-fagarine, and evoxine and the new alkaloid haplobine for which on the basis of spectral characteristics and a passage to the main alkaloid haplopine (7-hydroxy-4,8-dimethoxyfuranoquinoline) the structure of 7-(3′-chloromethylbut-2′-enyloxy)-4,8-dimethoxyfuranoquinoline has been established.
Similar content being viewed by others
Literature cited
I. A. Bessonova, D. Kurbanov, and S. Yu. Yunusov, Khim. Prir. Soedin., 124 (1984).
R. A. W. Johnstone, Mass Spectrometry for Organic Chemists, Cambridge University Press (1972); R. M. Silverstein, G. C. Bassler, and T. Morrill, Spectrometric Identification of Organic Compounds, 3rd edn., Wiley, New York (1974).
A. Ya. Revo, Qualitative Microchemical Reactions in Organic Chemistry [in Russian], Moscow (1965), p. 28.
I. A. Bessonova and S. Yu. Yunusov, Khim. Prir. Soedin., 303 (1977).
K. C. Engvild, Phytochemistry,25, 781 (1986).
J. Reisch, Zs. Rozsa, K. Szendrei, I. Novak, and E. Minker, Phytochemistry,11, 2359 (1972);16, 151 (1977).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 736–737, November–December, 1986.
Rights and permissions
About this article
Cite this article
Bessonova, I.A., Yunusov, S.Y. Alkaloids of the roots of theHaplophyllum obtusifolium . Chem Nat Compd 22, 684–686 (1986). https://doi.org/10.1007/BF00598351
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00598351