Abstract
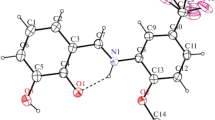
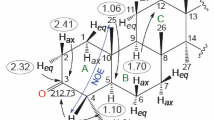
The results are given of a study of the stereochemistry of a coumarin terpenoid derivative — latilobinol — by nuclear magnetic resonance spectroscopy using lanthanoid shift reagents. On the basis of the results obtained, it has been established that the hydroxy group in the cyclohexane ring occupies the equatorial position.
Similar content being viewed by others
Literature cited
A. Z. Abyshev, Khim. Prir. Spedin., 90 (1979).
C. C. Hinckley, J. Am. Chem. Soc.,91, 5160 (1969).
J. M. Armitage and D. Hall, J. Am. Chem. Soc.,95, 1437 (1973).
C. C. Hinckley, M. R. Klotz, and F. Patil, J. Am. Chem. Soc.,93, 2417 (1971).
J. K. M. Saunders and D. H. Williams, J. Am. Chem. Soc.,93, 641 (1971).
L. Fieser and M. Fieser, Steroids, Reinhold, New York (1959).
A. I. Saidkhodzhaev, Khim. Prir. Soedin., 4 (1979).
Additional information
Leningrad Sanitary-Rygienic Medical Institute. Translated from Khimiya Prirodnykh Soedinenii, No.6, pp. 712–716, November–December, 1984.
Rights and permissions
About this article
Cite this article
Abyshev, A.Z. Stereochemistry of latilobinol. Chem Nat Compd 20, 674–677 (1984). https://doi.org/10.1007/BF00580019
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00580019