Abstract
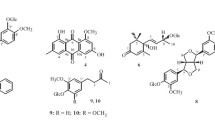
From the ligulate flowers ofLeucanthemum vulgare Lam. growing on the territory of the Georgian SSR a new glycoside has been isolated which has been called nivyaside and has the structure 8-(1-α-D-glucopyranosyl-5-deoxyquercit-5-yl)-4′,5,7-trihydroxyflavone.
Similar content being viewed by others
Literature cited
T. A. Geissman, The Chemistry of Flavonoid Compounds, Pergamon, Oxford (1962).
E. G. Bryant, J. Am. Pharm. Assoc.,39, No. 8, 48 (1950).
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer, New York (1970).
V. A. Bandyukova and V. A. Yugin, Khim. Prir. Soedin., 1 (1981).
N. K. Kochetkov, A. F. Bochkov, V. A. Dmitriev, O. S. Chizhov, and V. N. Shibaev, Carbohydrate Chemistry [in Russian], Moscow (1967), p. 64.
H. Kiliani, Chem. Ber.,63, 2866 (1930).
K. F. Blinova and Betkhi Tkuan', Rast. Res.,13, No. 3, 466 (1977).
H. Kindl and O. Hoffman-Ostenhof, Phytochem.,6, 77 (1967).
I. Heilbron and H. M. Bunbury, Dictionary of Organic Compounds, 2nd edn. (1943–4)
I. M. Hais and K. Macek, Paper Chromatography, 3rd edn., Academic Press, New York (1963).
T. Posternak, D. Raymond, and W. Haerdi, Helv. Chim. Acta,38, 191 (1955).
S. A. Barker, E. J. Bourne, R. Stephens, and D. H. Whiffen, J. Chem. Soc., No. 12, 4211 (1954).
J. P. Kukh, Anal. Chem.,22, No. 2, 276 (1950).
T. G. Sagareishvili, M. D. Alaniya, and É. P. Kemertelidze, Khim. Prir. Soedin., 567 (1980).
L. I. Deryugina, P. E. Krivenchuk, and G. P. Maksyutina, Khim. Prir. Soedin., 394 (1966).
S. I. Angyal and G. G. Macdonald, J. Chem. Soc., No. 2, 686 (1952).
N. P. Maksyutina and V. I. Litvinenko, Phenolic Compounds and Their Biological Functions [in Russian], Moscow (1968), p. 7.
I. P. Kovalev and V. I. Litvinenko, Khim. Prir. Soedin., 233 (1965).
I. Heilbron and H. M. Bunbury, Dictionary of Organic Compounds, 2nd edn. (1943–1944).
R. Kuhn and J. Löw, Chem. Ber.,77, 202 (1944).
M. D. Alaniya, N. F. Komissarenko and É. P. Kemertelidze, Izv. Akad. Nauk SSSR, Ser. Khim., No. 2, 1 (1976).
Additional information
I. G. Kutateladze Institute of Pharmacochemistry, Academy of Sciences of the Georgian SSR, Tbilisi. Translated from Khimiya Prirodnykh Soedinenii, No. 4, pp. 442–446, July–August, 1982.
Rights and permissions
About this article
Cite this article
Sagareishvili, T.G., Alaniya, M.D., Kikoladze, V.S. et al. Nivyaside — A new glycoside fromLeucanthemum vulgare . Chem Nat Compd 18, 408–412 (1982). https://doi.org/10.1007/BF00579632
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00579632