Abstract
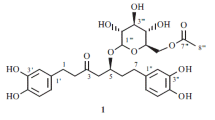
By chemical and spectral methods using INDOR the structure of an acylated coumarin glycoside, haploperoside B, isolated fromHaplophyllum perforatum has been established as 7-[2-0-[4-acetyl-α-L-rhamnopyranosyl)-β-D-glucopyranosyloxy]-6-methoxy-coumarin.
Similar content being viewed by others
Literature cited
M. P. Yuldashev, E. Kh. Batirov, and V. M. Malikov, Khim. Prir. Soedin., 412 (1980).
M. P. Yuldashev, E. Kh. Batirov, and V. M. Malikov, Khim. Prir. Soedin., 168 (1980).
B. Coxon, in: Methods in Carbohydrate Chemistry, Vol. 6, R. L. Whistler and J. N. BeMiller, eds., Academic Press, New York (1972), pp. 513–545.
R. U. Lemieux, R. K. Kulling, H. J. Bernstein, and W. G. Schneider, J. Am. Chem. Soc.,79, 1005 (1957);80, 6098 (1958).
J. F. Stoddart, Stereochemistry of Carbohydrates, Interscience, New York (1971).
G. Kotowycz and R. U. Lemieux, Chem. Rev.,73, 669 (1973).
L. D. Hall, Adv. Carbohydrate Chem.,19, 51 (1964).
H. Okabe and T. Kawasaki, Chem. Pharm. Bull.,20, 514 (1972).
D. Brown, R. O. Asplund, and V. A. McMahon, Phytochemistry,13, 1923 (1974).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. All-Union Scientific-Research Institute of Medicinal Plants, Moscow. Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 718–721, November–December, 1981.
Rights and permissions
About this article
Cite this article
Yuldashev, M.P., Batirov, E.K., Malikov, V.M. et al. The structure of haploperoside B — An acylated coumarin glycoside fromHaplophyllum perforatum . Chem Nat Compd 17, 518–520 (1981). https://doi.org/10.1007/BF00574368
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00574368