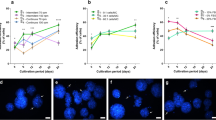
Abstract
For the purpose of establishing a large scale production process of biologically active substances by cultivation of anchorage-dependent mammalian cells, basic studies were carried out on the following items; establishment of a new cell line and derivation of high productivity; construction of optimal serum-free medium; optimization of cultivation method using microcarrier in serum-free medium; and establishment of purification process. The cell line, TRC-29SF, used in this study was newly established from human renal carcinoma with a function of producing macrophage colony-stimulating factor constitutively. Improvement of M-CSF productivity upon TRC-29SF cell line was performed by M-CSF gene amplification with dhfr-MTX system and by truncation of membrane-binding amino acid sequence by recombinant DNA technique. Two kinds of serum-free media, IPEG-85 and IREG-89, were formulated for the growth of TRC-29SF cell and its transformant, respectively. A new cell-adhesion method which permits homogeneous attachment to microcarrier in short term was developed by equalising the sedimentation velocity between cells and microcarrier by addition of 7% Ficoll into the medium. High cell density perfusion culture of TRC-29SF cells was achieved by microcarrier method using IPEG-85 medium, and final cell density reached over 107 cells/ml. Based on the results obtained, long-term perfusion cultures were performed using Mn10-5 and Mn10-5/R600 cell lines, which were created by M-CSF gene transfection and amplification. We found that the productivity of M-CSF per cell began to decrease from the end of logarithmic growth phase. Long-term cultivation with high productivity was accomplished by perfusing medium containing 2 mM sodium butyrate. Purification process for M1-CSF from the culture supernatant of transformed cell line was also established.
Similar content being viewed by others
Abbreviations
- CSF:
-
Colony-Stimulating Factor
- M-CSF:
-
Macrophage Colony-Stimulating Factor
- EGF:
-
Epidermal Growth Factor
- FCS:
-
Fetal Calf Serum
- dhfr:
-
dihydrofolate reductase
- MTX:
-
Methotrexate
- HEPES:
-
N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid)
- KLa:
-
oxygen mass transfer coefficient
- cDNA:
-
complementary DNA
- CAT:
-
Chloramphenicol Acetyltransferase
- pfu:
-
plaque forming unit
- KDa:
-
Kilo-daltons
References
BarnesD and SatoG (1980a) Methods for growth of cultured cells in serum free medium. Anal. Biochem. 102: 255–270.
BarnesD and SatoG (1980b) Serum-free cell culture: A unifying approach. Cell 22: 649–655.
BoosmanA, StricklerJ and WilsonK (1987) Partial primary structures of human and murine macrophage colony stimulating factor (CSF-1). Biochem. Biophys. Res. Commun. 144: 74–80.
BoshartM, WeberF, JahnG, Dorsch-HaslerK, FleckensteinB and SchaffnerW (1985) A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41: 521–530.
BottensteinJ, HayashiI, HutchingsS, MasuiH, MatherJ, McClureDB, OhasaS, RizzinoA, SatoG, SerreroG, WolfeR and WuR (1979) The growth of cells in serum-free hormone-supplemented media. Methods Enzymol. 58: 94–109.
BurrowsMT (1924) Relation of oxygen to the growth of tissue cells. Am. J. Physiol. 68: 110 (abstract).
ButlerM, ImamuraT, ThomasJ and ThillyWG (1983) High yields from microcarrier cultures by medium perfusion. J. Cell Sci. 61: 351–363.
GassonCG, WeibartRH, KaufmanSE, ClarkSC, HewichRM, WongGG and GolbeDW (1984) Purified human granulocyte-macrophage colony-stimulating factor: Direct action on neutrophils. Science 226: 1339–1342.
GentryLE, WebbNR, LimGJ, BrunnerAM, RanchalisJE, TwardzikDR, LioubinMN, MarquardtH and PurchioAF (1987) Type I transforming growth factor beta: Amplified expression and secretion of mature and precursor polypeptides in Chinese hamster ovary cells. Mol. Cell Biol. 7: 3418–3427.
GluzmanY (1981) SV40-trasformed simian cells support the replication of early SV40 mutants. Cell 23: 175–182.
GormanCM, MoffatLF and HowardBH (1982) Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2: 1044–1051.
GraffS and McCartyKS (1957) Sustained cell culture. Exp. Cell Res. 13: 348–457.
GrifinBE (1981) In: ToozeJ (eds.) DNA tumor viruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp. 61–123.
GublerU and HoffmanBJ (1983) A simple and very efficient method for generating cDNA libraries. Gene 25: 263–269.
HayashiI and SatoGH (1976) Replacement of serum by hormones permits growth of cells in a defined medium. Nature 259: 132–134.
HimmelfarbP, ThayerPS and MartinHE (1969) Spin filter culture: The propagation of mammalian cells in suspension. Science 164: 555–557.
JayeM, HowkR, BurgessW, RiccaGA, ChiuIM, RaveraMW, O'BrienSJ, ModiWS, MaciagT and DrohanWN (1986) Human endotherial cell growth factor: Cloning, nucleotide sequence, and chromosome localization. Science 233: 541–548.
JensenMD, WallachDFH and LinPS (1974) Comparative growth characteristics of Vero cells on gas-permeable and conventional supports. Expl. Cell Res. 84: 271–281.
JonesM and BontingSL (1956) Some relations between growth and carbohydrate metabolism in tissue cultures. Exp. Cell Res. 10: 631–639.
KashiwaguraT, WilsonDF and ErecinskaM (1984) Oxygen dependence of cellular metabolism: The effect of O2-tension on gluconeogenesis. J. Cell. Physiol. 120: 13–18.
KawasakiES, LandnerMB, WangAM, ArsdellJV, WarrenMK, CoyneMY, SchweickartVL, LeeMT, WilsonKJ, BoosmanA, StanleyER and MarkDF (1985) Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science 230: 293–296.
KnazekRA, GullinoPM, KohlerPO and DedrickRL (1972) Cell culture on artficial capillaries: An approach to tissue growthin vitro. Science 178: 65–67.
KnopRH, ChenCW, MitchellJB, RussoA, McPhersonS and CohenJS (1984) Metabolic studies of mammalian cells by31P-NMR using a continuous perfusion technique. Biochim. Biophys. Acta 804: 275–284.
KrebsHA (1950) Body size and tissue respiration. Biochim. Biophys. Acta 4: 249–269.
KumazakiT, TerasawaK and IshiiS (1987) Affinity chromatography on immobilized anhydrotrypsin: General utility for selective isolation of C-terminal peptides from protease digests of proteins. J. Biochem. 102: 1539–1546.
LederLD (1987) Der Blutmonocyt. Springer Berlin-Heidelberg-New York, p. 223.
ManosMM (1988) Expression and processing of a recombinant human macrophage colony-stimulating factor in mouse cells. Mol. Cell. Biol. 8: 5035–5039.
McCormickF, TraheyM, InnisM, DiekmannB and RingoldG (1984) Inducible expresslon of amplified human beta interferon genes in CHO cells. Mol. Cell. Biol. 4: 166–172.
McLimansWF, BlumensonLE and TunnahKV (1968) Kinetics of gas diffusion in mammalian cell culture systems. II. Theory. Biotechnol. Bioeng. 10: 741–763.
MolonyWB, McPhersonK and FliegelmanL (1980) Esterase activity in leukocytes demonstrated by the use of naphtol AS-D chloroacetate substrate. J. Histochem. Cytochem. 8: 200.
OnomichiK, EtoY and ShibaiH (1987) Expression of amplified cDNA sequences encoding human interleukin 2 in human cells. Agric. Biol. Chem. 51: 1385–1392.
PikeBL and RobinsonWA (1970) Human bone marrow colony growth in agar-gel. J. Cell Physiol. 78: 77–84.
RamabhadranTV, ReitzBA and ShahDM (1985) Highlevel expression of the bovine growth hormone gene in heterologous mammalian cells. Gene 38: 111–118.
RaskL, AnudiH and PetersonPA (1979) The primary structure of human retinol-binding protein. FEBS. Lett. 104: 55–58.
RettenmierCW, RousselMF, AshmunRA, RalphP, PriceK and SherrCJ (1987) Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol. Cell. Biol. 7: 2378–2387.
RichterA, SanfordKK and EvansVJ (1972) Influence of oxygen and culture media on plating efficiency of some mammalian tissue cells. J. Natn. Cancer Inst. 49: 1705–1712.
SakaiN, UmedaT, SuzukiH, IshimatsuY and ShikitaM (1987) Macrophage colony-stimulating factor purified from normal human urine. 222: 341–344.
SangerF and CoulsonAR (1978) The use of thin acrylamide gel for DNA sequencing. FEBS Lett. 87: 107–110.
Sato GH (1975) The role of serum in cell culture. In: Litwack G (ed.) Biochemical actions of hormones. Vol. 3 pp. 391-396.
Satoh S, Kobayashi J and Otani M (1990) Isolation and characterization of a growth-promoting factor from calf serum, and identification to retinol-binding protein.In Vitro in press.
Satoh S and Mizoguchi J (1989) Improved extracellular secretion of M-CSF by deleting transmembrane region of the molecule. Cell Culture Engineering II Abst., Santa Barbara, CA, p. 7.
SchahillSJ, DevosR, Van derheydenJ and FiersW (1983) Expression and characterization of the product of a human immune interferon cDNA gene in chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA 80: 4654–4658.
SchwalzelF and BrannsteinH (1968) Cytochemishe Darstellung von Esteraseactivaten in Blutund Knochenmarkszellen. Klin. Wschr. 48: 642.
SimonsenCC and LevinsonAD (1983) Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc. Natl. Acad. Sci. USA 80: 2495–2499.
SourthernPJ and BergP (1982) Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J. Mol. Appl. Genet. 1: 327–341.
SouzaLM, BooneTC, GagriloveJ, LaiPH, ZseboKM, MurdockDC, ChazinVR, BruszewskiJ, LuH, ChenKK, BarendtJ, PlatzerE, MooreMAS, MetelsmannR and WelteK (1986) Recombinant human granulocyte colonystimulating factor: Effects on normal and leukemic myeloid cells. Science 232: 61–65.
SpierRE and GriffithsB (1983) An examination of the data and concepts germane to the oxygenation of cultured animal cells. Dev. Biol. Stand. 55: 81–92.
StevensKM (1965) Oxygen requirements for liver cellsin vitro. Nature 206: 199.
SundelinJ, LaurentBC, AnudiH, TragardhL, LarhammarD, BjorckL, ErikssonU, AkerstromB, JonesA, NewcomerM, PetersonPA and RaskL (1985) Amino acid sequence homologies between rabbit, rat, and human serum retinolbinding proteins. J. Biol. Chem. 260: 6472–6480.
TempletonD and EckhartW (1984) N-terminal amino acid sequence of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol. Cell. Biol. 4: 817–821.
TsuneokaK and ShikitaM (1985) A colony-stimulating factor for neutrophil granulocytes: A marked increase of its production by addition of sodium butyrate and lipopolysaccharide in serum-free culture of RSP-2·P3 cells. J. Cell. Physiol. 125: 436–442.
UrlaubG and ChasinLA (1980) Isolation of chinese hamster cell mutants deficient in dehydrofolate reductase activity. Proc. Natl. Acad. Sci. USA 77: 4216–4220.
WerrleinRJ and GlinosAD (1974) Oxygen microenvironment and respiratory oscillations in cultured mammalian cells. Nature 251: 371–379.
WongG, TemplePA, LearyAC, Witek-GiannottiJS, YangYC, CiarlettaAB, ChungM, MurthaP, KrizR, KaufmanRJ, FerenzCR, SibleyBS, TurnerKJ, HewickRM, ClarkSC, YanaiN, YokotaH, YamadaM, SaitoM, MotoyosiK and TakakuF (1987) Human CSF-1: Molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science 235: 1504–1508.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Satoh, S., Kobayashi, J., Mizoguchi, J. et al. Serum-free cultivation of anchorage-dependent cells on microcarrier: Effective production of human macrophage colony-stimulating factor. Cytotechnology 5 (Suppl 2), 95–114 (1991). https://doi.org/10.1007/BF00573882
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00573882