Conclusions
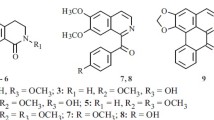
1. The alkaloids perforine and haplophyllidine isolated from the seeds ofH. perforatum have the developed formulas C16H17N(OH)2(OCH3)2(-O-) and C16H16N(OH) (OCH3)2(-O-).
2. Perforine is 7-hydroxy-8-(3′-hydroxy-3′-methylbutyl)-4, 8-dimethoxy-5, 6, 7, 8-tetrahydrofuroquinoline, and haplophyllidine is 7-hydroxy-4, 8-dimethoxy-(3′-methyl-2′-butenyl)-5, 6, 7, 8-tetrahydrofuroquinoline.
3. The transition from perforine to haplophyllidine has been effected by the splitting off of hydrogen chloride from chloroacetylperforine.
Similar content being viewed by others
References
G. P. Sidyakin, I. A. Bessonova, and S. Yu. Yunusov, DAN UzSSR, no. 10, 33, 1959.
T. T. Shakirov, G. P. Sidyakin, and S. Yu. Yunusov, DAN UzSSR, no. 6, 28, 1959; no. 9, 40, 1960; no. 8, 47, 1961.
L. H. Briggs and L. D. Colebrook, J. Chem. Soc., 2458, 1960.
Houben-Weyl, Methoden der organischen Chemie [Russian translation], Moscow, vol. 2, p. 260, 1963.
Alfred W. Sangster and Kenneth L. Stuart, Chem. Rev.65, 69, 1965.
O. E. Edwards and N. F. Elmore, Can. J. Chem.,40, 256, 1962.
A. V. Robertson, Austr. J. Chem.,16, 451, 1963.
L. Bellamy, Infrared Spectra of Complex Molecules [Russian translation], Moscow, p. 267, 1963.
N. Bhacca and D. Williams, Applications of NMR Spectroscopy in Organic Chemistry [Russian translation], Moscow, 1966.
S. L. Portnova, Yu. N. Sheinker, A. A. Akhrem, A. M. Prokhoda, and A. V. Kamernitskii, ZhOrKh,3, 44, 1967.
P. R. Jones, Usp. Khim.,35, 1589, 1966, [Russian translation of Chem. Revs.63, 461, 1963].
R. H. F. Manske and H. L. Holmes, The Alkaloids: Chemistry and Physiology, New York,3, 72, 1953.
T. J. Batternam and J. A. Lamberton, Austr. J. Chem.,18, 859, 1965.
J. Houben, Methoden der organischen Chemie [Russian translation], Moscow, vol. 3, part 3, 1935.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 4, No. 6, pp. 360–367, 1968
Rights and permissions
About this article
Cite this article
Faizutdinova, Z.S., Bessonova, I.A. & Yunusov, S.Y. The structure of perforine and haplophyllidine. Chem Nat Compd 4, 304–309 (1968). https://doi.org/10.1007/BF00569810
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00569810