Abstract
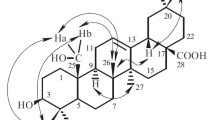
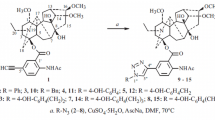
The roots ofFerula lapidosa Eug.Korov. have yielded lapidin, C20H30O4, mp 80–81°C (hexane), [α]D20 +166° (c 1.5; chloroform) — an ester of a new carotane alcohol lapidol and angelic acid. A structure and absolute configuration has been suggested for it on the basis of chemical transformations and spectral characteristics.
Similar content being viewed by others
Literature cited
G. V. Sagitdinova, A. I. Saidkhodzhaev, G. K. Nikonov, and U. Rakhmankulov, Khim. Prir. Soedin., 115 (1975).
G. V. Sagitdinova, A. I. Saidkhodzhaev, and V. M. Malikov, Khim. Prir. Soedin., 161 (1979).
M. C. Sriraman, B. A. Nagasampagi, R. C. Pandey, and Sukh Dev, Tetrahedron,29, 985 (1973).
A. I. Saidkhodzhaev, and G. K. Nikonov, Khim. Prir. Soedin., 559 (1972); 28 (1973); 166 (1974); 105 (1976).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR, Tashkent. Translated from Khimiya Prirodnykh Soedenii, No. 3, pp. 318–323, May–June, 1981.
Rights and permissions
About this article
Cite this article
Golovina, L.A., Saidkhodzhaev, A.I. Structure and stereochemistry of lapidin. Chem Nat Compd 17, 244–248 (1981). https://doi.org/10.1007/BF00568511
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00568511