Summary
1. About ten substances of a flavonoid nature have been found in the leaves ofPhellodendron sachalinense (F. Schm.) Sarg. andPh. amurense Rupr.
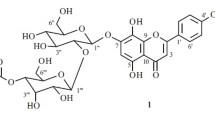
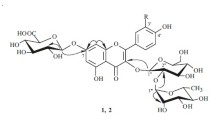
2. The two species of cork tree each contain two new flavonoids, which have been called phelloside and dihydrophelloside. The results of chemical and spectroscopic studies have permitted phelloside to be characterized as the 7,γ-di-O-β-D-glucopyranoside of noricaritin and dihydrophelloside as the 7,γ-di-O-β-D-glucopyranoside of dihydronoricaritin, the base of which contains kaempferol and aromadendrin.
3. Amurensin (the 7-β-D-glucopyranoside of noricaritin) and icariside-1 (the λ-O-β-D-glucopyranoside of noricaritin) have been isolated as intermediates in the stepwise hydrolysis of phelloside; the latter has not been previously found in the Amur and Japanese cork trees.
Similar content being viewed by others
References
M. Hasegawa, T. Shirato, J. Amer. Chem. Soc., 75, 5507, 1953.
T. Bodalski and E. Lamer, Dissert. Pharmaceut., 15, 319, 1963.
T. Bodalski and E. Lamer, Dissert. Pharmaceut., 16, 67, 1964.
R. M. Horowitz, J. Org. Chem., 22, 1733, 1957.
L. Hörhammer and K. H. Müller, Arch. Pharm., 287, 310, 1954.
L. Hurd, Spectral Properties of Flavonoid Compounds, in The Chemistry of Flavonoid Compounds, ed. T. A. Geissman, Pergamon Press, N. Y., 107, 1962.
V. I. Litvinenko, N. P. Maksyutina, and D. G. Kolesnikov, ZhOKh, 33, 4014, 1963.
V. I. Litvinenko and N. P. Maksyutina, KhPS [Chemistry of Natural Compounds], 420, 1965.
J. W. Clark-Lewis, Rev. Pure and Appl. Chem., 12, 96, 1962.
M. M. Pashchenko, G. P. Pivnenko, and V. I. Litvinenko, Farm. Zh. (Kiev), 21, no. 1, 44, 1966.
S. Akai, J. Pharm. Soc., Japan, 55, 112, 1935.
V. I. Litvinenko, Rastitel'nye Resursy, 2, no. 4, 65, 1966.
Author information
Authors and Affiliations
Additional information
Khimiya Prirodnykh Soedinenii, Vol. 4, No. 2, pp. 77–82, 1968
Rights and permissions
About this article
Cite this article
Shevchuk, O.I., Maksyutina, N.P. & Litvinenko, V.I. Flavonoids ofPhellodendron sachalinense andPh. amurense . Chem Nat Compd 4, 66–70 (1968). https://doi.org/10.1007/BF00568013
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00568013