Abstract
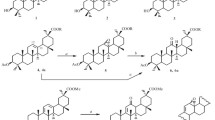
The composition of the products of the oxymercuration-demercuration reaction of car-3-ene according to the degree of its transformation has been studied. It has been established that this reaction forms trans-caran-3-ol, α-terpineol, m-menth-6-en-8-ol, 3,4-epoxy-trans-carane, p- and m-1, 8-cineoles, p- and m-1,8-terpins, and terpene esters. It has been shown that the double bond of car-3-ene possesses a higher reactivity than the cyclopropane ring, and the opening of the latter takes place equally at both external bonds.
Similar content being viewed by others
Literature cited
B. A. Arbuzov, V. V. Ratner, Z. G. Isaeva, and É.Kh. Kazakova, Izv. Akad. Nauk SSSR, Ser. Khim., 385 (1972).
J. Simonsen, The Terpenes, Cambridge University Press, Vol. 2 (1949), p. 65; L. Borowiecki, Rocz. Chem.,39, No. 39, 257 (1965); I. I. Bardyshev, Zh. F. Loiko, L. V. Sionskaya, and R. G. Shlyashinskii, Dokl. Akad. Nauk BelorussSSR,10, 873 (1966); B. A. Arbuzov, V. V. Ratner, and Z. G. Isaeva, Izv. Akad. Nauk SSSR, Ser. Khim., 45 (1973).
R. Silverstein, G. Bassler, and T. Morrill, Spectrometric Identification of Organic Compounds, 2nd ed., Wiley, New York (1967).
R. A. Kretchmer, R. A. Conrad, and E. D. Mihelich, J. Org. Chem.,38, 1251 (1973).
H. C. Brown and P. J. Geoghegan, J. Am. Chem. Soc.,89, 1522 (1967).
B. A. Arbuzov, Z. G. Isaeva, and I. B. Nemirovskaya, Izv. Akad. Nauk SSSR, Ser. Khim., 1410 (1969).
I. I. Bardyshev and I. V. Gorbacheva, in; Synthetic Products from Rosin and Turpentine [in Russian], Gorkii (1970), p. 190.
R. Lombard and G. Ambroise, Bull. Soc. Chim. Fr., No. 2, 230 (1961).
J. M. Coxon, M. P. Hartshorn, J. W. Mitchel, and K. E. Richards, Chem. Ind. (London), 652 (1968).
M. Goryaev and I. Pliva, Methods of Investigating Essential Oils [in Russian], Alma-Ata (1962), p. 301.
W. Cocker, P. Shannon, and P. Staniland, J. Chem. Soc., C, No. 1, 41 (1966).
K. Gollnik, S. Shroeter, O. Ohloff, G. Schade, and G. Schenk, Ann. Chem.,687, 14 (1965).
Y.-R. Naves, Helv. Chim. Acta,31, No. 44, 48 (1948); V. Paolini, Gazz. Chim. Ital.,55, 810 (1925).
Additional information
Institute of Physical Organic Chemistry of the Academy of Sciences of the Belorussian SSR, Minsk. Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 646–650, September–October, 1979.
Rights and permissions
About this article
Cite this article
Buinova, É.F., Yaremchenko, N.G., Urbanovich, T.R. et al. Oxymercuration-demercuration of car-3-ene. Chem Nat Compd 15, 565–568 (1979). https://doi.org/10.1007/BF00565925
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565925