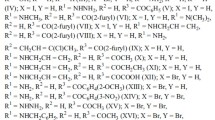Summary
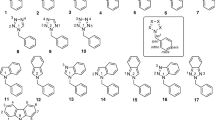
The use of the NOE method has permitted the unambiguous determination of the position of the OCH3 groups at C10 in the aromatic nuclei of majoridine and of O-acetyl-N-methyllochnerine and has thereby shown the possibility of using this method for determining the position of substituents in the benzene rings of the N-methylindoline and indole alkaloids.
Similar content being viewed by others
Literature cited
F. R. Chalmers, H. T. Openshaw, and G. F. Smith, J. Chem. Soc., 1115 (1957).
M. F. Millson and S. R. Robinson, J. Chem. Soc., 3362 (1955).
M. M. Janot and J. L. Men, Compt. Rend.,240, 909 (1955).
J. A. Joule and G. F. Smith, J. Chem. Soc., 312 (1962).
A. Hogman and A. J. Trey, Helv. Chim. Acta,40, 1866 (1957).
M. Hanaoka, M. Hesse, and H. Schmid, Helv. Chim. Acta,53, 1723 (1970).
H. Meisel and W. Döpke, Tetrahedron Lett.,17, 1285 (1971).
J. L. Kaul, J. Trojanek, and A. K. Bose, Collection Czech. Chem. Commun.,35, 116 (1970).
P. Kh. Yuldashev, J. L. Kaul, Z. Kablitsova, J. Trojanek, and S. Yu. Yunusov, Khim. Prirodn. Soedin.,2, 192 (1966).
A. Stoll and A. Hoffman, Helv. Chim. Acta,36, 1143 (1953).
W. Mors, P. Zaltman, J. Beereboon, S. Pakzashi, and C. Djerassi, Chem. Ind. (London), 173 (1956).
N. Peube-Locou, M. Koch, M. Plat, and P. Potier, Phytochemistry,11, 2109 (1972).
S. Sakai, A. Kubo, and L. Haginiwa, Tetrahedron Lett., 1485 (1969).
W. Klyne, R. J. Swan, N. J. Dastoor, A. A. Gorman, and H. Schmid, Helv. Chim. Acta,50, 115 (1967).
K. Warnat, Helv. Chim. Acta,14, 997 (1931).
R. A. Bell and J. K. Saunders, Can. J. Chem.,48, 1114 (1970).
G. E. Bachers and T. Schaefer, Chem. Rev.,71, No. 6, 617 (1971).
M. R. Yagudaev, V. M. Malikov, and S. Yu. Yunusov, Khim. Prirodn. Soedin.,9, 70 (1973).
P. J. Black, R. D. Brown, and M. L. Heffernan, Austr. J. Chem.,20, 1325 (1967).
Additional information
Institute of the Chemistry of Plant Substances, Academy of Sciences of the Uzbek SSR. Translated from Khimiya Prirodnykh Soedinenii, No. 3, pp. 316–319, May–June, 1973.
Rights and permissions
About this article
Cite this article
Yagudaev, M.R., Malikov, V.M. & Yunusov, S.Y. The use of the intramolecular nuclear Overhauser effect to establish the positions of substituents in the aromatic nuclei of the alkaloids majoridine and O-acetyl-N-methyllochnerine. Chem Nat Compd 9, 302–304 (1973). https://doi.org/10.1007/BF00565686
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565686