Abstract
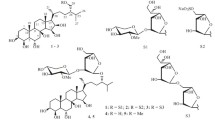
The total sterols have been isolated fromHalocynthia aurantium by column chromatography on silica gel. The following steroid alcohols have been identified in it with the aid of GLC, GLC-MS, and1H NMR: 5α-cholestan-3β-ol, 24ξ-methylcholestan-3β-ol, 24ξ-ethylcholestan-3β-ol, 4ξ-methyl-24ξ-ethyl-5α-cholestan-3β-ol, cholest-5-en-3β-ol, 24ξ-methylcholest-5-en-3β-ol, 24ξ-ethylcholes-5-en-3β-ol, 5α-cholest-22-en-3β-ol, 24-nor-5α-cholest-22-en-3β-ol, cholesta-5,22-dien-3β-ol, 24ξ-methylcholesta-5,22-dien-3β-ol, 24-norcholesta-5,22-dien-3β-ol, 24-ethylcholesta-5,24(28)-dien-3β-ol, and 24-methylcholesta-5,24(28)-dien-3β-ol.
Similar content being viewed by others
Literature cited
L. K. Shubina, V. A. Stonik, and G.B. Elyakov, Khim. Prirodn. Soedin., 104 (1981).
D. R. Idler and L. M. Safe, Steroids,19, 315 (1972).
S. Teshima, A. Kanazawa, and T. Ando, Mem. Fac. Fish, Kagoshima Univ.,22, 7 (1973).
R. Rihage and E. Stenhagen, J. Lipid Res.,1, 361 (1960).
F. F. Knapp and G. J. Schroepfer, Steroids,26, 339 (1975).
B. A. Knight, J. Gas Chromatogr.,5, 273 (1967).
S. G. Willie and C. Djerassi, J. Org. Chem.,28, 305 (1968).
T. R. Erdman and R. H. Thomson, Tetrahedron,28, 5163 (1972).
D. R. Idler, P. Wiseman, and L. M. Safe, Steroids,16, 451 (1970).
P. J. Scheuer, Marine Natural Products, Academic Press, New York, Vol. I (1978), p. 253.
J. L. Boutry, A. Saliot, and M. Barbier, Experientia,35, 1541 (1979).
J. A. Ballantine, A. Lavis, J. C. Roberts, and R. J. Morris, J. Exp. Mar. Biol. Ecol.,30, 29 (1977).
G. W. Patterson, Anal. Chem.,43, 1165 (1971).
R. C. Reitz and J. G. Hamilton, Comp. Biochem. Physiol.,25, 401 (1968).
S. Yasuda, Comp. Biochem. Physiol.,44B, 41 (1973).
M. Kobayashi and H. Mitsuhashi, Steroids26, 605 (1975).
Additional information
Pacific Ocean Institute of Bioorganic Chemistry of the Far Eastern Scientific Center of the Academy of Sciences of the USSR, Vladivostok, and Far-Eastern State University, Vladivostok. Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 581–585, September–October, 1981.
Rights and permissions
About this article
Cite this article
Shubina, L.K., Moskovkina, T.V., Stonik, V.A. et al. Steroid alcohols from the ascidianHalocynthia aurantium . Chem Nat Compd 17, 418–422 (1981). https://doi.org/10.1007/BF00565153
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565153