Abstract
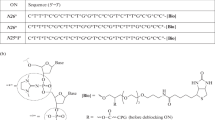
A method is proposed for determining the position of guanosine residues in oligo-deoxyribonucleotides. After limited modification of the oligonucleotide with glyoxal, a mixture of molecules of the initial oligonucleotide with different degrees of modification is formed. In the presence of borate ions, the glyoxal-modified guanosine residues form negatively charged borate complexes which inhibit the action of snake venom phosphodiesterase. The treatment of such a complex with the enzyme forms a mixture of fragments of the initial oligonucleotide the 5′-terminal sequences of which are identical while at the 3′-end there are modified guanosine residues. The determination of the length of these fragments provides the necessary information on the positions of the guanosine residues in the chain of the initial oligonucleotides. The positions of the guanosine residues in eight synthetic oligodeoxyribonucleotides have been confirmed with the aid of the method developed.
Similar content being viewed by others
Literature cited
G. G. Brownlee and F. Sanger, Eur. J. Biochem.,11, 395 (1969).
V. Ling, J. Mol. Biol.,64, 87 (1972).
A. Maxam and W. Gilbert, Proc. Nat. Acad. Sci. U.S.A.,74, 560 (1977).
E. D. Sverdlov, G. S. Monastyrskaya, A. V. Chestukhin, and E. I. Budowsky, FEBS Lett.,33, 15 (1973).
E. D. Sverdlov, G. S. Monastyrskya, E. I. Budowsky, and M. A. Grachev, FEBS Lett.,28, 231 (1972).
E. D. Sverdlov, G. S. Monastyrskaya, and É. I. Budovskii, Mol. Biol.,11, 116 (1977).
V. G. Korobko, S. A. Grachev, and N. A. Petrov, Bioorg. Khim.,3, 1423 (1977).
V. G. Metelev, V. D. Smirnov, Z. A. Shabarova, and E. D. Sverdlov, FEBS Lett.,90, 112 (1978).
E. Yu. Krynetskii, V. G. Metelev, V. D. Smirnov, and Z. A. Shabarova, Khim. Prir. Soedin., 253 (1978).
E. A. Ivanova, I. I. Kolodkina, and A. M. Yurkevich, Zh. Obshch. Khim.,41, 455 (1971).
S. V. Kuz'min, in: D. G. Knorre and T. V. Venkstern (eds.), The Ultramicroanalysis of Nucleic Acids [in Russian], Moscow (1973), pp. 95–103.
Additional information
A. N. Belozerskii Interfaculty Problem Scientific-Research Laboratory of Molecular Biology and Bioorganic Chemistry of the M. V. Lomonosov Moscow State University. Translated from Khimiya Prirodnykh Soedinenii, No. 4, pp. 570–573, July–August, 1979.
Rights and permissions
About this article
Cite this article
Tuneev, V.M., Metelev, V.G. & Shabarova, Z.A. Method of determining the position of guanosine residues in oligodeoxyribonucleotides. Chem Nat Compd 15, 495–498 (1979). https://doi.org/10.1007/BF00565055
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00565055



